Fibroblast growth factor receptor 1 (FGFR1) tyrosine phosphorylation regulates binding of FGFR substrate 2alpha (FRS2alpha) but not FRS2 to the receptor
- PMID: 17901128
- PMCID: PMC2194630
- DOI: 10.1210/me.2007-0140
Fibroblast growth factor receptor 1 (FGFR1) tyrosine phosphorylation regulates binding of FGFR substrate 2alpha (FRS2alpha) but not FRS2 to the receptor
Abstract
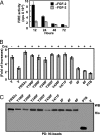
Binding of the fibroblast growth factor (FGF) to the FGF receptor (FGFR) tyrosine kinase leads to receptor tyrosine autophosphorylation as well as phosphorylation of multiple downstream signaling molecules that are recruited to the receptor either by direct binding or through adaptor proteins. The FGFR substrate 2 (FRS2) family consists of two members, FRS2alpha and FRS2beta, and has been shown to recruit multiple signaling molecules, including Grb2 and Shp2, to FGFR1. To better understand how FRS2 interacted with FGFR1, in vivo binding assays with coexpressed FGFR1 and FRS2 recombinant proteins in mammalian cells were carried out. The results showed that the interaction of full-length FRS2alpha, but not FRS2beta, with FGFR1 was enhanced by activation of the receptor kinase. The truncated FRS2alpha mutant that was comprised only of the phosphotyrosine-binding domain (PTB) bound FGFR1 constitutively, suggesting that the C-terminal sequence downstream the PTB domain inhibited the PTB-FGFR1 binding. Inactivation of the FGFR1 kinase and substitutions of tyrosine phosphorylation sites of FGFR1, but not FRS2alpha, reduced binding of FGFR1 with FRS2alpha. The results suggest that although the tyrosine autophosphorylation sites of FGFR1 did not constitute the binding sites for FRS2alpha, phosphorylation of these residues was essential for optimal interaction with FRS2alpha. In addition, it was demonstrated that the Grb2-binding sites of FRS2alpha are essential for mediating signals of FGFR1 to activate the FiRE enhancer of the mouse syndecan 1 gene. The results, for the first time, demonstrate the specific signals mediated by the Grb2-binding sites and further our understanding of FGF signal transmission at the adaptor level.
Figures



References
-
- Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J 1997 A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693–702 - PubMed
-
- Ong SH, Goh KC, Lim YP, Low BC, Klint P, Claesson-Welsh L, Cao X, Tan YH, Guy GR 1996 Suc1-associated neurotrophic factor target (SNT) protein is a major FGF-stimulated tyrosine phosphorylated 90-kDa protein which binds to the SH2 domain of GRB2. Biochem Biophys Res Commun 225:1021–1026 - PubMed
-
- McDougall K, Kubu C, Verdi JM, Meakin SO 2001 Developmental expression patterns of the signaling adapters FRS-2 and FRS-3 during early embryogenesis. Mech Dev 103:145–148 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

