Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7
- PMID: 17901130
- PMCID: PMC5419630
- DOI: 10.1210/me.2006-0478
Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7
Abstract
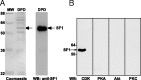
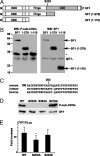
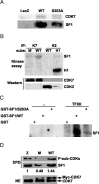
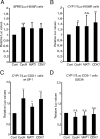
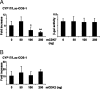
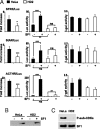
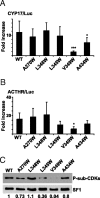
The nuclear receptor steroidogenic factor-1 (SF1) is critical for development and function of steroidogenic tissues. Posttranslational modifications are known to influence the transcriptional capacity of SF1, and it was previously demonstrated that serine 203 is phosphorylated. In this paper we report that serine 203 is phosphorylated by a cyclin-dependent kinase 7 (CDK7)-mediated process. As part of the CDK-activating kinase complex, CDK7 is a component of the basal transcription factor TFIIH, and phosphorylation of SF1 as well as SF1-dependent transcription was clearly reduced in cells carrying a mutation that renders the CDK-activating kinase complex unable to interact with the TFIIH core. Coimmunoprecipitation analyses revealed that SF1 and CDK7 reside in the same complex, and kinase assays demonstrated that immunoprecipitated CDK7 and purified TFIIH phosphorylate SF1 in vitro. The CDK inhibitor roscovitine blocked phosphorylation of SF1, and an inactive form of CDK7 repressed the phosphorylation level and the transactivation capacity of SF1. Structural studies have identified phosphoinositides as potential ligands for SF1. Interestingly, we found that mutations designed to block phospholipid binding dramatically decreased the level of SF1 phosphorylation. Together our results suggest a connection between ligand occupation and phosphorylation and association with the basic transcriptional machinery, indicating an intricate regulation of SF1 transactivation.
Figures

Similar articles
-
Varicella-zoster virus IE63 protein phosphorylation by roscovitine-sensitive cyclin-dependent kinases modulates its cellular localization and activity.J Biol Chem. 2005 Aug 12;280(32):29135-43. doi: 10.1074/jbc.M503312200. Epub 2005 Jun 13. J Biol Chem. 2005. PMID: 15955820
-
RNA polymerase II C-terminal heptarepeat domain Ser-7 phosphorylation is established in a mediator-dependent fashion.J Biol Chem. 2010 Jan 1;285(1):188-96. doi: 10.1074/jbc.M109.046565. Epub 2009 Nov 9. J Biol Chem. 2010. PMID: 19901026 Free PMC article.
-
Stimulation of RAR alpha activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7.Cell. 1997 Jul 11;90(1):97-107. doi: 10.1016/s0092-8674(00)80317-7. Cell. 1997. PMID: 9230306
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
Secrets of a double agent: CDK7 in cell-cycle control and transcription.J Cell Sci. 2005 Nov 15;118(Pt 22):5171-80. doi: 10.1242/jcs.02718. J Cell Sci. 2005. PMID: 16280550 Review.
Cited by
-
Hedgehog signaling and steroidogenesis.Annu Rev Physiol. 2015;77:105-29. doi: 10.1146/annurev-physiol-061214-111754. Annu Rev Physiol. 2015. PMID: 25668018 Free PMC article. Review.
-
A novel variant of NR5A1, p.R350W implicates potential interactions with unknown co-factors or ligands.Front Endocrinol (Lausanne). 2023 Jan 20;13:1033074. doi: 10.3389/fendo.2022.1033074. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36743925 Free PMC article.
-
Adrenocortical stem cells in health and disease.Nat Rev Endocrinol. 2025 Aug;21(8):464-481. doi: 10.1038/s41574-025-01091-2. Epub 2025 Mar 10. Nat Rev Endocrinol. 2025. PMID: 40065108 Review.
-
Coordination of Multiple Cellular Processes by NR5A1/Nr5a1.Endocrinol Metab (Seoul). 2020 Dec;35(4):756-764. doi: 10.3803/EnM.2020.402. Epub 2020 Dec 23. Endocrinol Metab (Seoul). 2020. PMID: 33397036 Free PMC article. Review.
-
Synergistic activation of the Mc2r promoter by FOXL2 and NR5A1 in mice.Biol Reprod. 2010 Nov;83(5):842-51. doi: 10.1095/biolreprod.110.085621. Epub 2010 Jul 21. Biol Reprod. 2010. PMID: 20650879 Free PMC article.
References
-
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP 2002. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res 57:19–36 - PubMed
-
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA 2005. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 - PubMed
-
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE 2005. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell 17:491–502 - PubMed
-
- Urs AN, Dammer E, Sewer MB 2006. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology 147:5249–5258 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

