Dissecting RNA chaperone activity
- PMID: 17901153
- PMCID: PMC2080586
- DOI: 10.1261/rna.671807
Dissecting RNA chaperone activity
Abstract
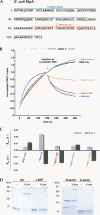
Many RNA-binding proteins help RNAs to fold via their RNA chaperone activity. This term has been used widely without accounting for the diversity of the observed reactions, which include complex events like restructuring of misfolded catalytic RNAs, promoting the assembly of RNA-protein complexes, and mediating RNA-RNA interactions. Proteins display very diverse activities depending on the assays used to measure RNA chaperone activity. To classify proteins with this activity, we compared three exemplary proteins from E. coli, host factor Hfq, ribosomal protein S1, and the histone-like protein StpA for their abilities to promote two simple reactions, RNA annealing and strand displacement. The results of a FRET-based assay show that S1 promotes only RNA strand displacement while Hfq solely enhances RNA annealing. StpA, in contrast, is active in both reactions. To test whether the two activities can be assigned to different domains of the bipartite-structured StpA, we assayed the purified N- and C- terminal domains separately. While both domains are unable to promote RNA annealing, we can attribute the RNA strand displacement activity of StpA to the C-terminal domain. Correlating with their RNA annealing activities, only Hfq and full-length StpA display simultaneous binding of two RNAs, suggesting a matchmaker-like model for this activity. For StpA, this "RNA crowding" requires protein-protein interactions, since a dimerization-deficient StpA mutant lost the ability to bind and anneal two RNAs. These results underline the difference between the two reaction types, making it necessary to distinguish and classify proteins according to their specific RNA chaperone activities.
Figures

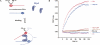
Similar articles
-
RNA chaperone activity and RNA-binding properties of the E. coli protein StpA.Nucleic Acids Res. 2007;35(4):1257-69. doi: 10.1093/nar/gkl1143. Epub 2007 Jan 31. Nucleic Acids Res. 2007. PMID: 17267410 Free PMC article.
-
Domain structure and RNA annealing activity of the Escherichia coli regulatory protein StpA.Mol Microbiol. 1998 May;28(4):847-57. doi: 10.1046/j.1365-2958.1998.00848.x. Mol Microbiol. 1998. PMID: 9643551
-
Structural and biochemical studies on ATP binding and hydrolysis by the Escherichia coli RNA chaperone Hfq.PLoS One. 2012;7(11):e50892. doi: 10.1371/journal.pone.0050892. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226421 Free PMC article.
-
New molecular interactions broaden the functions of the RNA chaperone Hfq.Curr Genet. 2019 Dec;65(6):1313-1319. doi: 10.1007/s00294-019-00990-y. Epub 2019 May 18. Curr Genet. 2019. PMID: 31104083 Review.
-
Proteins That Chaperone RNA Regulation.Microbiol Spectr. 2018 Jul;6(4):10.1128/microbiolspec.rwr-0026-2018. doi: 10.1128/microbiolspec.RWR-0026-2018. Microbiol Spectr. 2018. PMID: 30051798 Free PMC article. Review.
Cited by
-
The novel cis-encoded small RNA h2cR is a negative regulator of hfq2 in Burkholderia cenocepacia.PLoS One. 2012;7(10):e47896. doi: 10.1371/journal.pone.0047896. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082228 Free PMC article.
-
Defining a role for Hfq in Gram-positive bacteria: evidence for Hfq-dependent antisense regulation in Listeria monocytogenes.Nucleic Acids Res. 2010 Jan;38(3):907-19. doi: 10.1093/nar/gkp1081. Epub 2009 Nov 26. Nucleic Acids Res. 2010. PMID: 19942685 Free PMC article.
-
Rapid binding and release of Hfq from ternary complexes during RNA annealing.Nucleic Acids Res. 2011 Jul;39(12):5193-202. doi: 10.1093/nar/gkr062. Epub 2011 Mar 4. Nucleic Acids Res. 2011. PMID: 21378124 Free PMC article.
-
Role of RNA chaperones in virus replication.Virus Res. 2009 Feb;139(2):253-66. doi: 10.1016/j.virusres.2008.06.015. Epub 2008 Aug 8. Virus Res. 2009. PMID: 18675859 Free PMC article. Review.
-
The LARP6 La module from Tetrabaena socialis reveals structural and functional differences from plant and animal LARP6 homologues.RNA Biol. 2025 Dec;22(1):1-9. doi: 10.1080/15476286.2025.2489303. Epub 2025 Apr 9. RNA Biol. 2025. PMID: 40181506 Free PMC article.
References
-
- Bloch, V., Yang, Y., Margeat, E., Chavanieu, A., Auge, M.T., Robert, B., Arold, S., Rimsky, S., Kochoyan, M. The H-NS dimerization domain defines a new fold contributing to DNA recognition. Nat. Struct. Biol. 2003;10:212–218. - PubMed
-
- Blumenthal, T., Carmichael, G.G. RNA replication: Function and structure of Qβ-replicase. Annu. Rev. Biochem. 1979;48:525–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
