MAP kinase phosphatase activity sets the threshold for thymocyte positive selection
- PMID: 17901205
- PMCID: PMC2042194
- DOI: 10.1073/pnas.0705321104
MAP kinase phosphatase activity sets the threshold for thymocyte positive selection
Abstract
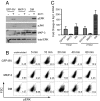
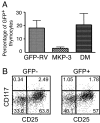
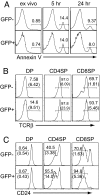
Phosphorylation of MAP kinases is important for proper translation of T cell antigen receptor (TCR) signals into thymocyte cell fates, but the role of MAP kinase phosphatase (MKP) activity in thymocyte development has not been characterized. To explore the role of MKP in thymocytes, we constructed a double mutant MKP-3 (DM-MKP3) that acts as a dominant-negative inhibitor of ERK- and JNK-specific MKP. Thymocytes developing in the presence of DM-MKP3 have enhanced frequencies of both CD4 and CD8 mature, single-positive cells and no increase in apoptosis. Expression of DM-MKP3 also results in an increased proportion of thymocytes with high levels of both CD69 and TCRbeta, suggesting that the increased proportion of mature thymocytes is the result of an increased probability that CD4(+)CD8(+) cells will be positively selected. Thus, MKP activity controls thymocyte cell fate by regulating the threshold of TCR signaling that is able to induce positive selection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

