The prefrontal cortex and flexible behavior
- PMID: 17901261
- PMCID: PMC2855184
- DOI: 10.1177/1073858407301369
The prefrontal cortex and flexible behavior
Abstract
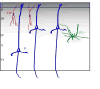
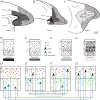
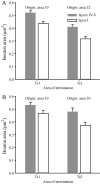
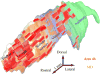
The prefrontal cortex in primates guides behavior by selecting relevant stimuli for the task at hand, mediated through excitatory bidirectional pathways with structures associated with sensory processing, memory, and emotions. The prefrontal cortex also has a key role in suppressing irrelevant stimuli through a mechanism that is not well understood. Recent findings indicate that prefrontal pathways interface with laminar-specific neurochemical classes of inhibitory neurons in sensory cortices, terminate extensively in the frontal and sensory sectors of the inhibitory thalamic reticular nucleus, and target the inhibitory intercalated masses of the amygdala. Circuit-based models suggest that prefrontal pathways can select relevant signals and efficiently suppress distractors, in processes that are disrupted in schizophrenia and in other disorders affecting prefrontal cortices.
Figures






References
-
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. - PubMed
-
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci. 2003;15:600–609. - PubMed
-
- Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol. 1986;252:415–422. - PubMed
-
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. - PubMed
-
- Barbas H. Complementary role of prefrontal cortical regions in cognition, memory and emotion in primates. Adv Neurol. 2000;84:87–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

