FA2H is responsible for the formation of 2-hydroxy galactolipids in peripheral nervous system myelin
- PMID: 17901466
- PMCID: PMC2662131
- DOI: 10.1194/jlr.M700400-JLR200
FA2H is responsible for the formation of 2-hydroxy galactolipids in peripheral nervous system myelin
Abstract
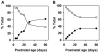
Myelin in the mammalian nervous system has a high concentration of galactolipids [galactosylceramide (GalCer) and sulfatide] with 2-hydroxy fatty acids. We recently reported that fatty acid 2-hydroxylase (FA2H), encoded by the FA2H gene, is the major fatty acid 2-hydroxylase in the mouse brain. In this report, we show that FA2H also plays a major role in the formation of 2-hydroxy galactolipids in the peripheral nervous system. FA2H mRNA and FA2H activity in the neonatal rat sciatic nerve increased rapidly during developmental myelination. The contents of 2-hydroxy fatty acids were approximately 5% of total galactolipid fatty acids at 4 days of age and increased to 60% in GalCer and to 35% in sulfatides at 60 days of age. The chain length of galactolipid fatty acids also increased significantly during myelination. FA2H expression in cultured rat Schwann cells was highly increased in response to dibutyryl cyclic AMP, which stimulates Schwann cell differentiation and upregulates myelin genes, such as UDP-galactose:ceramide galactosyltransferase and protein zero. These observations indicate that FA2H is a myelination-associated gene. FA2H-directed RNA interference (RNAi) by short-hairpin RNA expression resulted in a reduction of cellular 2-hydroxy fatty acids and 2-hydroxy GalCer in D6P2T Schwannoma cells, providing direct evidence that FA2H-dependent fatty acid 2-hydroxylation is required for the formation of 2-hydroxy galactolipids in peripheral nerve myelin. Interestingly, FA2H-directed RNAi enhanced the migration of D6P2T cells, suggesting that, in addition to their structural role in myelin, 2-hydroxy lipids may greatly influence the migratory properties of Schwann cells.
Figures



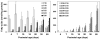
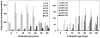

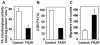
Similar articles
-
FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain.J Lipid Res. 2006 Dec;47(12):2772-80. doi: 10.1194/jlr.M600362-JLR200. Epub 2006 Sep 23. J Lipid Res. 2006. PMID: 16998236
-
Myelination in the absence of UDP-galactose:ceramide galactosyl-transferase and fatty acid 2 -hydroxylase.BMC Neurosci. 2011 Mar 2;12:22. doi: 10.1186/1471-2202-12-22. BMC Neurosci. 2011. PMID: 21366909 Free PMC article.
-
The human FA2H gene encodes a fatty acid 2-hydroxylase.J Biol Chem. 2004 Nov 19;279(47):48562-8. doi: 10.1074/jbc.M406649200. Epub 2004 Aug 27. J Biol Chem. 2004. PMID: 15337768
-
Galactolipids are molecular determinants of myelin development and axo-glial organization.Biochim Biophys Acta. 2002 Dec 19;1573(3):406-13. doi: 10.1016/s0304-4165(02)00410-5. Biochim Biophys Acta. 2002. PMID: 12417425 Review.
-
2'-Hydroxy ceramide in membrane homeostasis and cell signaling.Adv Biol Regul. 2014 Jan;54:223-30. doi: 10.1016/j.jbior.2013.09.012. Epub 2013 Oct 8. Adv Biol Regul. 2014. PMID: 24139861 Free PMC article. Review.
Cited by
-
Functional investigation of SLC1A2 variants associated with epilepsy.Cell Death Dis. 2022 Dec 21;13(12):1063. doi: 10.1038/s41419-022-05457-6. Cell Death Dis. 2022. PMID: 36543780 Free PMC article.
-
Defective lipid metabolism in neurodegeneration with brain iron accumulation (NBIA) syndromes: not only a matter of iron.J Inherit Metab Dis. 2015 Jan;38(1):123-36. doi: 10.1007/s10545-014-9770-z. Epub 2014 Oct 10. J Inherit Metab Dis. 2015. PMID: 25300979 Review.
-
Plant sphingolipid fatty acid 2-hydroxylases have unique characters unlike their animal and fungus counterparts.Plant Signal Behav. 2012 Nov;7(11):1388-92. doi: 10.4161/psb.21825. Epub 2012 Aug 23. Plant Signal Behav. 2012. PMID: 22918503 Free PMC article.
-
Gene Expression Profile in the Sandhoff Mouse Brain with Progression of Age.Genes (Basel). 2022 Nov 3;13(11):2020. doi: 10.3390/genes13112020. Genes (Basel). 2022. PMID: 36360256 Free PMC article.
-
Soluble Neuregulin1 Down-Regulates Myelination Genes in Schwann Cells.Front Mol Neurosci. 2018 May 14;11:157. doi: 10.3389/fnmol.2018.00157. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29867349 Free PMC article.
References
-
- Norton WT, Cammer W. In: Myelin. Morell P, editor. Plenum; New York: 1984. pp. 147–195.
-
- Hoshi M, Williams M, Kishimoto Y. Characterization of brain cerebrosides at early stages of development in the rat. J Neurochem. 1973;21:709–712. - PubMed
-
- Kishimoto Y, Radin NS. Isolation and determination methods for brain cerebrosides, hydroxy fatty acids, and unsaturated and saturated fatty acids. J Lipid Res. 1959;1:72–78.
-
- Boggs JM, Koshy KM, Rangaraj G. Influence of structural modifications on the phase behavior of semi- synthetic cerebroside sulfate. Biochim Biophys Acta. 1988;938:361–372. - PubMed
-
- Lofgren H, Pascher I. Molecular arrangements of sphingolipids. The monolayer behaviour of ceramides. Chem Phys Lipids. 1977;20:273–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

