Studies of anandamide accumulation inhibitors in cerebellar granule neurons: comparison to inhibition of fatty acid amide hydrolase
- PMID: 17901541
- PMCID: PMC2248273
- DOI: 10.1007/s12031-007-0045-0
Studies of anandamide accumulation inhibitors in cerebellar granule neurons: comparison to inhibition of fatty acid amide hydrolase
Abstract
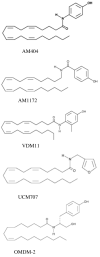
The endocannabinoid, N-arachidonylethanolamine (AEA) is accumulated by neurons via a process that has been characterized biochemically but not molecularly. Inhibitors of AEA accumulation have been characterized individually but have not been compared in a single study. Our purpose was to compare the potency of five previously described compounds (AM404, AM1172, VDM11, OMDM-2, and UCM707) both as inhibitors of AEA and N-palmitoylethanolamine (PEA) accumulation by cerebellar granule neurons and as inhibitors of AEA hydrolysis. The compounds all inhibited AEA accumulation; AM404, VDM11 and OMDM-2 with IC(50) values of approximately 5 microM, whereas AM1172 and UCM707 exhibited IC(50) values of 24 and 30 microM, respectively. The compounds also inhibited PEA accumulation; AM404 being the most potent with an IC(50) of 6 microM, whereas the other compounds had IC(50) values in the range of 30-70 microM. All of the compounds potently inhibited AEA hydrolysis by brain membranes; the K(I) values for AM404, VDM11, and UCM707 were less than 1 microM; AM1172 and OMDM-2 exhibited K(I) values of 3 and 10 microM, respectively. The IC(50) values for inhibition of AEA accumulation were compared to the IC(50) values for PEA accumulation and AEA hydrolysis using linear regression. None of the regressions were significant. These data indicate that inhibition of AEA accumulation by neurons is not a result of the inhibition of endocannabinoid hydrolysis and is a process different from the accumulation of PEA. These studies support the hypothesis that the cellular AEA accumulation beyond simple equilibrium between intracellular and extracellular concentrations occurs because AEA binds to an intracellular protein that is not FAAH but that also recognizes the AEA uptake inhibitors.
Figures


References
-
- Beltramo M, di Tomaso E, Piomelli D. Inhibition of anandamide hydrolysis in rat brain tissue by (E)-6- (bromomethylene) tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one. FEBS Lett. 1997a;403:263–267. - PubMed
-
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997b;277:1094–1097. - PubMed
-
- Day TA, Rakhshan F, Deutsch DG, Barker EL. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol Pharmacol. 2001;59:1369–1375. - PubMed
-
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

