siRNA mediated the type 1 insulin-like growth factor receptor and epidermal growth factor receptor silencing induces chemosensitization of liver cancer cells
- PMID: 17901981
- PMCID: PMC12161626
- DOI: 10.1007/s00432-007-0314-x
siRNA mediated the type 1 insulin-like growth factor receptor and epidermal growth factor receptor silencing induces chemosensitization of liver cancer cells
Abstract
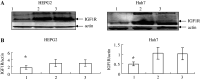
Purpose: This study was to investigate if downregulation of IGF1R and EGFR by RNA interference (RNAi) would sensitize human liver cancer cells (HEPG2, Huh7 ) to adriamycin.
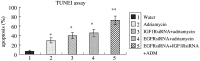
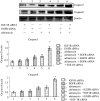
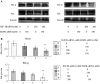
Methods: HEPG2, Huh7 cell lines were transfected IGF1R siRNAs and EGFR siRNAs and IGF1R or EGFR mRNA level was determined by RT-PCR and Western-blot analysis. We investigated the effects of the adriamycin-induced apoptosis of these cells by TUNEL assay. Also we analyze caspase3, 8 and the phosphorylation levels of Akt and Erk by Western-blot. The p53 effect of adriamycin-induced cell death by inhibitors of EGFR/IGF1R is investigated by cell growth curves.
Results: Transfection of an IGF1R and EGFR siRNAs resulted in substantial loss of IGF1R and EGFR mRNA of HEPG2, Huh7 cells relative to the control case. EGFR siRNA and IGF1R siRNA treatments increased the adriamycin-induced apoptosis of these cells. IGF1R siRNA and EGFR siRNA enhance a caspase-dependent cell death program. The phosphorylation levels of Akt and Erk were reduced by the combination of the two agents. The facilitation of adriamycin-induced cell death by inhibitors of EGFR/IGF1R is p53-independent.
Conclusions: The results indicate that the siRNA for IGF1R has a great potential for cancer therapy when combined with either a chemotherapeutic agent or siRNAs that targets EGFR.
Figures



References
-
- Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB (1979) Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature 282:615–616 - PubMed
-
- Albanell J, Baselga J (1996) Unraveling resistance to trastuzuMAb (Herceptin): insulin-like growth factor-I receptor, a new suspect. J Natl Cancer Inst 93:1830–1832 - PubMed
-
- Anderson NG, Ahmad T, Chan K, Dobson R, Bundred NJ (2001) ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFRpositive cancer cell lines with or without erbB2 overexpression. Int J Cancer 94:774–782 - PubMed
-
- Arteaga C (2002) Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist 7:31–39 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

