Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb
- PMID: 17904116
- PMCID: PMC2692258
- DOI: 10.1016/j.ydbio.2007.08.023
Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb
Abstract
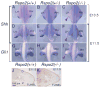
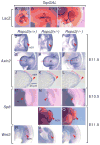
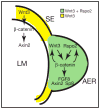
The R-spondin (Rspo) family of proteins consists of secreted cysteine-rich proteins that can activate beta-catenin signaling via the Frizzled/LRP5/6 receptor complex. Here, we report that targeted inactivation of the mouse Rspo2 gene causes developmental limb defects, especially in the hindlimb. Although the initiation of the expression of apical ectodermal ridge (AER)-specific genes, including fibroblast growth factor 8 (FGF8) and FGF4 occurred normally, the maintenance of these marker expressions was significantly defective in the hindlimb of Rspo2(-/-) mice. Consistent with the ligand role of R-spondins in the Wnt/beta-catenin signaling pathway, expression of Axin2 and Sp8, targets for beta-catenin signaling, within AER was greatly reduced in Rspo2(-/-) embryos. Furthermore, sonic hedgehog (Shh) signaling within the hindlimbs of Rspo2(-/-) mice was also significantly decreased. Rspo2 is expressed in the AER of all limb buds, however the stunted phenotype is significantly more severe in the hindlimbs than the forelimbs and strongly biased to the left side. Our findings strongly suggest that Rspo2 expression in the AER is required for AER maintenance likely by regulating Wnt/beta-catenin signaling.
Figures




References
-
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H. R-spondin3 is required for mouse placental development. Dev Biol. 2007;301:218–226. - PubMed
-
- Bergmann C, Senderek J, Anhuf D, Thiel CT, Ekici AB, Poblete-Gutierrez P, van Steensel M, Seelow D, Nurnberg G, Schild HH, Nurnberg P, Reis A, Frank J, Zerres K. Mutations in the gene encoding the Wnt-signaling component R-spondin 4 (RSPO4) cause autosomal recessive anonychia. Am J Hum Genet. 2006;79:1105–9. - PMC - PubMed
-
- Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM, Kelsell DP. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet. 2006;38:1245–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

