MDMA (N-methyl-3,4-methylenedioxyamphetamine) and its stereoisomers: Similarities and differences in behavioral effects in an automated activity apparatus in mice
- PMID: 17904622
- PMCID: PMC2994347
- DOI: 10.1016/j.pbb.2007.09.002
MDMA (N-methyl-3,4-methylenedioxyamphetamine) and its stereoisomers: Similarities and differences in behavioral effects in an automated activity apparatus in mice
Abstract
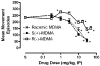
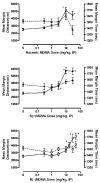
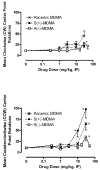
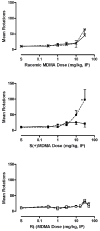
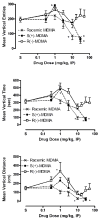
Racemic MDMA (0.3-30 mg/kg), S(+)-MDMA (0.3-30 mg/kg), R(-)-MDMA (0.3-50 mg/kg) and saline vehicle (10 ml/kg) were comprehensively evaluated in fully automated and computer-integrated activity chambers, which were designed for mice, and provided a detailed analysis of the frequency, location, and/or duration of 18 different activities. The results indicated that MDMA and its isomers produced stimulation of motor actions, with S(+)-MDMA and (+/-)-MDMA usually being more potent than R(-)-MDMA in measures such as movement (time, distance, velocity), margin distance, rotation (clockwise and counterclockwise), and retraced activities. Interestingly, racemic MDMA appeared to exert a greater than expected potency and/or an enhanced effect on measures such as movement episodes, center actions (entries and distance), clockwise rotations, and jumps; actions that might be explained by additive or synergistic (i.e. potentiation) effects of the stereoisomers. In other measures, the enantiomers displayed different effects: S(+)-MDMA produced a preference to induce counterclockwise (versus clockwise) rotations, and each isomer exerted a different profile of effect on vertical activities and jumps. Furthermore, each isomer of MDMA appeared to attenuate the effect of its opposite enantiomer on some behaviors; antagonism effects that were surmised from a lack of expected activities by racemic MDMA. S(+)-MDMA (but not R(-)-MDMA), for example, produced an increase in vertical entries (rearing) and a preference to increase counterclockwise (versus clockwise) rotations; (+/-)-MDMA also should have induced such effects but did not. Apparently, R(-)-MDMA, when combined with S(+)-MDMA to form (+/-)-MDMA, prevented the appearance of those increases (from control) in activities. Similarly, R(-)-MDMA (but not S(+)-MDMA) produced increases in episodes (i.e. jumps) and vertical distance that racemic MDMA also should have, but were not, exhibited. Evidently, the presence of S(+)-MDMA in the racemic mixture inhibited the appearance of those increases (from control) in behavior. Taken together, the various and complex effects of MDMA and its stereoisomers are noted and a strategy is suggested for future studies that stresses the importance of steric effects and interplay, probable interaction(s) with various neurotransmitters, and interaction(s) with the particular behavioral or biological event (or action) being measured.
Figures




Similar articles
-
(±)-MDMA and its enantiomers: potential therapeutic advantages of R(-)-MDMA.Psychopharmacology (Berl). 2018 Feb;235(2):377-392. doi: 10.1007/s00213-017-4812-5. Epub 2017 Dec 16. Psychopharmacology (Berl). 2018. PMID: 29248945 Review.
-
Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine ("ecstasy") and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice.Psychopharmacology (Berl). 2003 Mar;166(3):202-11. doi: 10.1007/s00213-002-1261-5. Epub 2003 Feb 1. Psychopharmacology (Berl). 2003. PMID: 12563544
-
Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse.Psychopharmacology (Berl). 2005 Sep;181(3):529-36. doi: 10.1007/s00213-005-0005-8. Epub 2005 Oct 12. Psychopharmacology (Berl). 2005. PMID: 15983787
-
Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice.Psychopharmacology (Berl). 2004 May;173(3-4):270-7. doi: 10.1007/s00213-003-1741-2. Epub 2004 Jan 22. Psychopharmacology (Berl). 2004. PMID: 14740148
-
Distribution of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) stereoisomers in a fatal poisoning.Forensic Sci Int. 1996 Dec 2;83(2):111-9. doi: 10.1016/s0379-0738(96)02025-7. Forensic Sci Int. 1996. PMID: 9022274 Review.
Cited by
-
Locomotor effects of 3,4-methylenedioxymethamphetamine (MDMA) and its deuterated form in mice: psychostimulant effects, stereotypy, and sensitization.Psychopharmacology (Berl). 2020 Feb;237(2):431-442. doi: 10.1007/s00213-019-05380-3. Epub 2019 Nov 15. Psychopharmacology (Berl). 2020. PMID: 31729537 Free PMC article.
-
A Systematic Review of the MDMA Model to Address Social Impairment in Autism.Curr Neuropharmacol. 2021;19(7):1101-1154. doi: 10.2174/1570159X19666210101130258. Curr Neuropharmacol. 2021. PMID: 33388021 Free PMC article.
-
Acute effects of R-MDMA, S-MDMA, and racemic MDMA in a randomized double-blind cross-over trial in healthy participants.Neuropsychopharmacology. 2024 Dec;50(2):362-371. doi: 10.1038/s41386-024-01972-6. Epub 2024 Aug 23. Neuropsychopharmacology. 2024. PMID: 39179638 Free PMC article. Clinical Trial.
-
Separating the agony from ecstasy: R(-)-3,4-methylenedioxymethamphetamine has prosocial and therapeutic-like effects without signs of neurotoxicity in mice.Neuropharmacology. 2018 Jan;128:196-206. doi: 10.1016/j.neuropharm.2017.10.003. Epub 2017 Oct 6. Neuropharmacology. 2018. PMID: 28993129 Free PMC article.
-
(±)-MDMA and its enantiomers: potential therapeutic advantages of R(-)-MDMA.Psychopharmacology (Berl). 2018 Feb;235(2):377-392. doi: 10.1007/s00213-017-4812-5. Epub 2017 Dec 16. Psychopharmacology (Berl). 2018. PMID: 29248945 Review.
References
-
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3,4- methylenedioxymethamphetamine on locomotor activity: role of 5-HT1B/1D and 5-HT2 receptors. Neuropsychopharmacology. 2002;26:40–52. - PubMed
-
- Barnett SA, Cowan PE. Activity, exploration, curiosity and fear: An ethological study. Interdiscip Sci Rev. 1976;1:43–62.
-
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mössner R, Westphal H, Lesch K-P. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. - PubMed
-
- Berlyne DE. Conflict, Arousal, and Curiosity. New York: McGraw-Hill; 1960.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

