In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide
- PMID: 17904680
- PMCID: PMC2211426
- DOI: 10.1016/j.jconrel.2007.08.029
In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide
Abstract
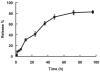
In the development of anti-cancer drugs, it is important to yield selective cytotoxicity primarily against tumor tissues. To achieve this goal, the use of a polymer-drug conjugate appears to be appealing, simply because it can take the advantage of the so-called enhanced permeability and retention (EPR) effect due to vascular leak in tumors. Among various types of polymers, polyrotaxane (PR) is an interesting candidate and warrants further consideration. It is a self-assembled polymer made entirely of biocompatible components, by threading alpha-cyclodextrin (alpha-CD) molecules with the poly(ethylene glycol) (PEG) chain. The abundance in functional -OH groups on the CD residues renders PR the capability of carrying a large dose of small anti-tumor agents for delivery. Herein, we presented a novel PR-based delivery system using doxorubicin (DOX) as the model anti-cancer drug. Daunorubicin (DNR) was conjugated to the PR polymer via hydrolysable linkages, and upon hydrolysis, doxorubicin was released as the cytotoxic drug. To facilitate an intracellular uptake by the tumor cells of the PR-DOX conjugates, a cell-penetrating low molecular weight protamine (LMWP) peptide was further attached to the two termini of the PR chain. Using an innovative principle established in our laboratory, such as via the inhibition of the cell-penetrating activity by binding with heparin and reversal of this inhibition by subsequent addition of protamine, cellular uptake of the polymer-drug conjugates could be readily regulated. In this paper, we performed in vitro studies to demonstrate the feasibility of this delivery system. The LMWP-PR-DOX conjugates, which yielded a sustained release of DOX over a period of greater than 4 days, were successfully synthesized. Intracellular uptake of these conjugates by A2780 human ovarian cancer cells and regulation of such uptake by heparin and protamine were confirmed by using the MTT assay and also the confocal microscopy method.
Figures





References
-
- Jain RK. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J Control Release. 2001;74(1–3):7–25. - PubMed
-
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. - PubMed
-
- Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74(1–3):47–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

