A prospective, randomized, multicenter trial of amnioreduction vs selective fetoscopic laser photocoagulation for the treatment of severe twin-twin transfusion syndrome
- PMID: 17904975
- PMCID: PMC2754290
- DOI: 10.1016/j.ajog.2007.07.020
A prospective, randomized, multicenter trial of amnioreduction vs selective fetoscopic laser photocoagulation for the treatment of severe twin-twin transfusion syndrome
Abstract
Objective: The objective of the study was to examine the effect of selective fetoscopic laser photocoagulation (SFLP) vs serial amnioreduction (AR) on perinatal mortality in severe twin-twin transfusion syndrome (TTTS).
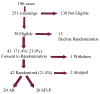
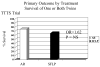
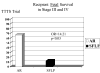
Study design: This was a 5 year multicenter, prospective, randomized controlled trial. The primary outcome variable was 30 day postnatal survival of donors and recipients.
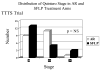
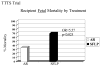
Results: There was no statistically significant difference in 30-day postnatal survival between SFLP or AR treatment for donors at 55% (11 of 20) vs 55% (11 of 20) (P = 1.0, odds ratio [OR] 1, 95% confidence interval [CI] 0.242 to 4.14) or recipients at 30% (6 of 20) vs 45% (9 of 20) (P = .51, OR 1.88, 95% CI 0.44 to 8.64). There was no difference in 30 day survival of 1 or both twins on a per-pregnancy basis between AR at 75% (15 of 20) and SFLP at 65% (13 of 20) (P = .73, OR 1.62, 95% CI 0.34 to 8.09). Overall survival (newborns divided by the number of fetuses treated) was not statistically significant for AR at 60% (24 of 40) vs SFLP 45% (18 of 40) (P = .18, OR 2.01, 95% CI 0.76 to 5.44). There was a statistically significant increase in fetal recipient mortality in the SFLP arm at 70% (14 of 20) vs the AR arm at 35% (7 of 20) (P = .25, OR 5.31, 95% CI 1.19 to 27.6). This was offset by increased recipient neonatal mortality of 30% (6 of 20) in the AR arm. Echocardiographic abnormality in recipient twin Cardiovascular Profile Score is the most significant predictor of recipient mortality (P = .055, OR 3.025/point) by logistic regression analysis.
Conclusion: The outcome of the trial did not conclusively determine whether AR or SFLP is a superior treatment modality. TTTS cardiomyopathy appears to be an important factor in recipient survival in TTTS.
Figures


Comment in
-
A randomized trial for the treatment of TTTS, too little to answer the question.Am J Obstet Gynecol. 2008 May;198(5):608-9; author reply 609. doi: 10.1016/j.ajog.2008.01.014. Epub 2008 Mar 24. Am J Obstet Gynecol. 2008. PMID: 18359474 No abstract available.
-
Power and interpretation of a randomized study on the treatment of severe twin-to-twin transfusion syndrome.Am J Obstet Gynecol. 2008 May;198(5):607; author reply 607-8. doi: 10.1016/j.ajog.2008.01.016. Am J Obstet Gynecol. 2008. PMID: 18455548 No abstract available.
-
The con trial.Am J Obstet Gynecol. 2009 Mar;200(3):e14-5; author reply e15. doi: 10.1016/j.ajog.2008.07.041. Epub 2008 Nov 6. Am J Obstet Gynecol. 2009. PMID: 18992859 No abstract available.
References
-
- Weir PE, Ratten GJ, Beischer NA. Acute polyhydramnios-a complication of monozygous twin pregnancy. Br J Obstet Gynaecol. 1979;86:849–53. - PubMed
-
- Steinberg LH, Hurley VA, Desmedt E, Beischer NA. Acute polyhydramnios in twin pregnancies. Austral NZ J Obstet Gynecol. 1990;30:196–200. - PubMed
-
- Cheschier NC, Seeds JW. Polyhydramnios and oligohydramnios in twin gestations. Obstet Gynecol. 1988;71:882–4. - PubMed
-
- Saunders NJ, Snijders RJ, Nicolaides KH. Twin-twin transfusion syndrome during the 2nd trimester is associated with small intertwin haemoglobin differences. Fetal Diag Ther. 1991;6:34–6. - PubMed
-
- Callahan TL, Hall JE, Ettner SL, Christiansen CL, Greene ML, Crowley WF. The economic impact of multiple-gestation pregnancies and the contribution of assisted-reproduction techniques to their incidence. N Engl J Med. 1994;331:244–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

