Folding and stability of the isolated Greek key domains of the long-lived human lens proteins gammaD-crystallin and gammaS-crystallin
- PMID: 17905830
- PMCID: PMC2211709
- DOI: 10.1110/ps.072970207
Folding and stability of the isolated Greek key domains of the long-lived human lens proteins gammaD-crystallin and gammaS-crystallin
Abstract
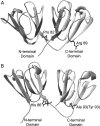
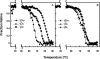
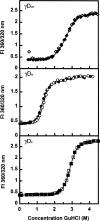
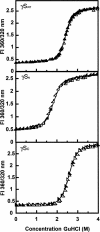
The transparency of the eye lens depends on the high solubility and stability of the lens crystallin proteins. The monomeric gamma-crystallins and oligomeric beta-crystallins have paired homologous double Greek key domains, presumably evolved through gene duplication and fusion. Prior investigation of the refolding of human gammaD-crystallin revealed that the C-terminal domain folds first and nucleates the folding of the N-terminal domain. This result suggested that the human N-terminal domain might not be able to fold on its own. We constructed and expressed polypeptide chains corresponding to the isolated N- and C-terminal domains of human gammaD-crystallin, as well as the isolated domains of human gammaS-crystallin. Both circular dichroism and fluorescence spectroscopy indicated that the isolated domains purified from Escherichia coli were folded into native-like monomers. After denaturation, the isolated domains refolded efficiently at pH 7 and 37 degrees C into native-like structures. The in vitro refolding of all four domains revealed two kinetic phases, identifying partially folded intermediates for the Greek key motifs. When subjected to thermal denaturation, the isolated N-terminal domains were less stable than the full-length proteins and less stable than the C-terminal domains, and this was confirmed in equilibrium unfolding/refolding experiments. The decrease in stability of the N-terminal domain of human gammaD-crystallin with respect to the complete protein indicated that the interdomain interface contributes of 4.2 kcal/mol to the overall stability of this very long-lived protein.
Figures

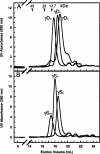
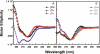
 ) γSN, and (orange
) γSN, and (orange  ) γSC.
) γSC.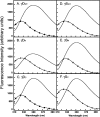


References
-
- Aarts H.J., Lubsen, N.H., and Schoenmakers, J.G. 1989. Crystallin gene expression during rat lens development. Eur. J. Biochem. 183: 31–36. - PubMed
-
- Bagby S., Go, S., Inouye, S., Ikura, M., and Chakrabartty, A. 1998. Equilibrium folding intermediates of a Greek key β-barrel protein. J. Mol. Biol. 276: 669–681. - PubMed
-
- Basak A., Bateman, O., Slingsby, C., Pande, A., Asherie, N., Ogun, O., Benedek, G.B., and Pande, J. 2003. High-resolution X-ray crystal structures of human γD crystallin (1.25 Å) and the R58H mutant (1.15 Å) associated with aculeiform cataract. J. Mol. Biol. 328: 1137–1147. - PubMed
-
- Bateman O.A., Lubsen, N.H., and Slingsby, C. 2001. Association behaviour of human βB1-crystallin and its truncated forms. Exp. Eye Res. 73: 321–331. - PubMed
-
- Bateman O.A., Sarra, R., van Genesen, S.T., Kappe, G., Lubsen, N.H., and Slingsby, C. 2003. The stability of human acidic β-crystallin oligomers and hetero-oligomers. Exp. Eye Res. 77: 409–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

