Reversal of cell polarity and actin-myosin cytoskeleton reorganization under mechanical and chemical stimulation
- PMID: 17905847
- PMCID: PMC2186262
- DOI: 10.1529/biophysj.107.114702
Reversal of cell polarity and actin-myosin cytoskeleton reorganization under mechanical and chemical stimulation
Abstract
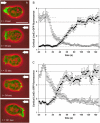
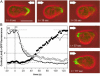
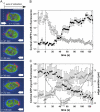
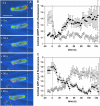
To study reorganization of the actin system in cells that invert their polarity, we stimulated Dictyostelium cells by mechanical forces from alternating directions. The cells oriented in a fluid flow by establishing a protruding front directed against the flow and a retracting tail. Labels for polymerized actin and filamentous myosin-II marked front and tail. At 2.1 Pa, actin first disassembled at the previous front before it began to polymerize at the newly induced front. In contrast, myosin-II slowly disappeared from the previous tail and continuously redistributed to the new tail. Front specification was myosin-II independent and accumulation of polymerized actin was even more focused in mutants lacking myosin-II heavy chains. We conclude that under mechanical stimulation, the inversion of cell polarity is initiated by a global internal signal that turns down actin polymerization in the entire cell. It is thought to be elicited at the most strongly stimulated site of the cell, the incipient front region, and to be counterbalanced by a slowly generated, short-range signal that locally activates actin polymerization at the front. Similar pattern of front and tail interconversion were observed in cells reorienting in strong gradients of the chemoattractant cyclic AMP.
Figures




References
-
- Hardin J., Walston T. Models of morphogenesis: the mechanisms and mechanics of cell rearrangement. Curr. Opin. Genet. Dev. 2004;14:399–406. - PubMed
-
- Dormann D., Weijer C.J. Chemotactic cell movement during Dictyostelium development and gastrulation. Curr. Opin. Genet. Dev. 2006;16:367–373. - PubMed
-
- Blaser H., Reichman-Fried M., Castanon I., Dumstrei K., Marlow F., Kawakami K., Solnica-Krezel L., Heisenberg C., Raz E. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev. Cell. 2006;11:613–627. - PubMed
-
- Gerisch G. Chemotaxis in Dictyostelium. Annu. Rev. Physiol. 1982;44:535–552. - PubMed
-
- Weber I., Wallraff E., Albrecht R., Gerisch G. Motility and substratum adhesion of Dictyostelium wild-type and cytoskeletal mutant cells: a study by RICM/bright-field double-view image analysis. J. Cell Sci. 1995;108:1519–1530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

