Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells
- PMID: 17905861
- PMCID: PMC2151705
- DOI: 10.1104/pp.107.103945
Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells
Abstract
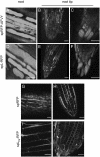
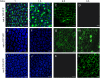
We generated fusions between three Arabidopsis (Arabidopsis thaliana) tonoplast intrinsic proteins (TIPs; alpha-, gamma-, and delta-TIP) and yellow fluorescent protein (YFP). We also produced soluble reporters consisting of the monomeric red fluorescent protein (RFP) and either the C-terminal vacuolar sorting signal of phaseolin or the sequence-specific sorting signal of proricin. In transgenic Arabidopsis leaves, mature roots, and root tips, all TIP fusions localized to the tonoplast of the central vacuole and both of the lumenal RFP reporters were found within TIP-delimited vacuoles. In embryos from developing, mature, and germinating seeds, all three TIPs localized to the tonoplast of protein storage vacuoles. To determine the temporal TIP expression patterns and to rule out mistargeting due to overexpression, we generated plants expressing YFP fused to the complete genomic sequences of the three TIP isoforms. In transgenic Arabidopsis, gamma-TIP expression was limited to vegetative tissues, but specifically excluded from root tips, whereas alpha-TIP was exclusively expressed during seed maturation. delta-TIP was expressed in vegetative tissues, but not root tips, at a later stage than gamma-TIP. Our findings indicate that, in the Arabidopsis tissues analyzed, two different vacuolar sorting signals target soluble proteins to a single vacuolar location. Moreover, TIP isoform distribution is tissue and development specific, rather than organelle specific.
Figures







Comment in
-
Multiple vacuoles in plant cells.Plant Physiol. 2008 Mar;146(3):1024-5; author reply 1026-7. doi: 10.1104/pp.107.900248. Plant Physiol. 2008. PMID: 18316646 Free PMC article. No abstract available.
References
-
- Brown JC, Jolliffe NA, Frigerio L, Roberts LM (2003) Sequence-specific, Golgi-dependent targeting of the castor bean 2S albumin to the vacuole in tobacco protoplasts. Plant J 36 711–719 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

