Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu
- PMID: 17906076
- PMCID: PMC2515112
- DOI: 10.1182/blood-2007-08-107292
Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu
Abstract
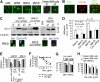
Currently, no approved monoclonal antibody (mAb) therapies exist for human multiple myeloma (MM). Here we characterized cell surface CS1 as a novel MM antigen and further investigated the potential therapeutic utility of HuLuc63, a humanized anti-CS1 mAb, for treating human MM. CS1 mRNA and protein was highly expressed in CD138-purified primary tumor cells from the majority of MM patients (more than 97%) with low levels of circulating CS1 detectable in MM patient sera, but not in healthy donors. CS1 was expressed at adhesion-promoting uropod membranes of polarized MM cells, and short interfering RNA (siRNA) targeted to CS1 inhibited MM cell adhesion to bone marrow stromal cells (BMSCs). HuLuc63 inhibited MM cell binding to BMSCs and induced antibody-dependent cellular cytotoxicity (ADCC) against MM cells in dose-dependent and CS1-specific manners. HuLuc63 triggered autologous ADCC against primary MM cells resistant to conventional or novel therapies, including bortezomib and HSP90 inhibitor; and pretreatment with conventional or novel anti-MM drugs markedly enhanced HuLuc63-induced MM cell lysis. Administration of HuLuc63 significantly induces tumor regression in multiple xenograft models of human MM. These results thus define the functional significance of CS1 in MM and provide the preclinical rationale for testing HuLuc63 in clinical trials, either alone or in combination.
Figures




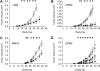
References
-
- Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. - PubMed
-
- Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831. - PubMed
-
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. - PubMed
-
- Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

