Autologous hematopoietic stem cell transplantation for high-risk brain tumors in children
- PMID: 17906911
- PMCID: PMC7100104
- DOI: 10.1007/s11060-007-9478-0
Autologous hematopoietic stem cell transplantation for high-risk brain tumors in children
Abstract
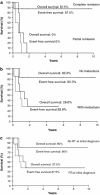
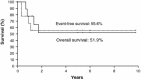
Autologous hematopoietic stem cell transplant (AHSCT) has been advocated as a form of salvage therapy for children with high-risk or relapsed brain tumors but only limited data are available currently. We report the outcomes of pediatric brain tumors treated with AHSCT in a quaternary referral center in Hong Kong over 10 years (June 1996-May 2006). Thirteen patients with medulloblastoma (n = 9), cerebral primitive neuroectodermal tumor (n = 1), ependymoma (n = 1), germ cell tumor (n = 1) and cerebellar rhabdoid (n = 1) were transplanted because of tumor residual (n = 1) or recurrence (n = 12). Uniform upfront treatment protocols were adopted according to specific tumor types. Prior to AHSCT, 8 patients (61.5%) achieved complete remission and 5 (38.5%) were in partial remission. Conditioning employed thiotepa 300 mg/m2, etoposide 250 mg/m2)and carboplatin 500 mg/m2 daily for 3 days. Toxicity included mucositis and neutropenic fever in all patients, grade 4 hepatic toxicity in 4 patients (including hepatic veno-occlusive disease in 2 patients) and grade 4 renal toxicity in 1 patient. The 5-year event-free survival was 53.9%. Five patients died of disease recurrence or progression 8-21 months after transplant with a median disease-free period of 8 months post-transplant. One died of transplant-related complications in the early post-transplant period. Seven survived for a median of 5.4 years (maximum follow-up of 9.8 years), with six having Lansky-Karnofsky performance score above 80. All survivors had complete remission before transplant though 2 had leptomeningeal spread. We conclude that AHSCT can achieve long-term survival in children with recurrent brain tumor. However, those with macroscopic residual tumor before transplant cannot be salvaged.
Figures
References
-
- Heideman RL, Cole DE, Balis F, et al. Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: evidence for dose-dependent plasma clearance of thiotepa. Cancer Res. 1989;49:736–741. - PubMed
-
- Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988;48:4189–4195. - PubMed
-
- Tartaglia RL, Cole DE, Heideman RL. Chemosensitivity of central nervous system tumors to thiotepa and tepa. Proc Am Assoc Cancer Res. 1989;30:461.
-
- Schold SC, Jr, Friedman HS, Bigner DD. Therapeutic profile of the human glioma line D-54 MG in athymic mice. Cancer Treat Rep. 1987;71:849–850. - PubMed
-
- Dunkel IJ, Boyett JM, Yates A, et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children’s Cancer Group. J Clin Oncol. 1998;16:222–228. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical