Bisphosphonate binding affinity as assessed by inhibition of carbonated apatite dissolution in vitro
- PMID: 17907244
- PMCID: PMC2743543
- DOI: 10.1002/jbm.a.31599
Bisphosphonate binding affinity as assessed by inhibition of carbonated apatite dissolution in vitro
Abstract
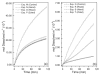
Bisphosphonates (BPs), which display a high affinity for calcium phosphate surfaces, are able to selectively target bone mineral, where they are potent inhibitors of osteoclast-mediated bone resorption. The dissolution of synthetic hydroxyapatite (HAP) has been used previously as a model for BP effects on natural bone mineral. The present work examines the influence of BPs on carbonated apatite (CAP), which mimics natural bone more closely than does HAP. Constant composition dissolution experiments were performed at pH 5.50, physiological ionic strength (0.15M) and temperature (37 degrees C). Selected BPs were added at (0.5 x 10(-6)) to (50.0 x 10(-6))M, and adsorption affinity constants, K(L), were calculated from the kinetics data. The BPs showed concentration-dependent inhibition of CAP dissolution, with significant differences in rank order zoledronate > alendronate > risedronate. In contrast, for HAP dissolution at pH 5.50, the differences between the individual BPs were considerably smaller. The extent of CAP dissolution was also dependent on the relative undersaturation, sigma, and CAP dissolution rates increased with increasing carbonate content. These results demonstrate the importance of the presence of carbonate in mediating the dissolution of CAP, and the possible involvement of bone mineral carbonate in observed differences in bone affinities of BPs in clinical use.
Figures






References
-
- de Jong WF. The mineral components of bones. Recueil des Travaux Chimiques des Pays-Bas et de la Belgique. 1926;45:445–448.
-
- Bigi A, Cojazzi G, Panzavolta S, Ripamonti A, Roveri N, Romanello M, Suarez KN, Moro L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J Inorg Biochem. 1997;68:45–51. - PubMed
-
- Astala R, Stott MJ. First principles investigation of mineral component of bone: CO3 substitutions in hydroxyapatite. Chem Mater. 2005;17:4125.
-
- Fleisch H. Bisphosphonates in Bone Disease: From the Laboratory to the Patient. San Diego, CA: Academic Press; 2000.
-
- LeGeros RZ. Calcium Phosphates in Oral Biology and Medicine. New York: Karger; 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

