Daytime pharmacodynamic and pharmacokinetic evaluation of low-dose sublingual transmucosal zolpidem hemitartrate
- PMID: 17907263
- PMCID: PMC2871168
- DOI: 10.1002/hup.884
Daytime pharmacodynamic and pharmacokinetic evaluation of low-dose sublingual transmucosal zolpidem hemitartrate
Abstract
Objectives: Buffered low-dose sublingual transmucosal zolpidem lozenge hemitartrate (ST zolpidem) is being developed for the treatment of middle-of-the-night insomnia. The objective of this double-blind placebo-controlled cross-over study (n = 24) was to evaluate the pharmacokinetics (PK) and daytime-sedative profile of 1.0, 1.75, and 3.5 mg dose of the formulation.
Methods: Daytime sedation was measured pre-dose and up to 5 h post-dose objectively by the Digit Symbol Substitution Test (DSST) and subjectively using the Visual Analog Scale (VAS). Blood samples for PK assessment was collected pre-dose and up to 12 h post-dose.
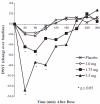
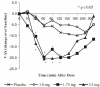
Results: The 1.75 and 3.5 mg, but not the 1 mg, ST zolpidem produced significant sedation versus placebo within 20 min of dosing which lasted for up to 3 h. Zolpidem from the formulation was rapidly absorbed and reached maximum plasma concentrations within 38 min of dosing, however the half-life was independent of the dose and side effects were consistent with the known pharmacology of the drug.
Conclusions: ST zolpidem produced rapid, short duration of sedation and the effect was consistent with its PK profile. This novel low-dose formulation of zolpidem may provide clinicians and patients with a prn option for the management of sleep maintenance insomnia.
(c) 2007 John Wiley & Sons, Ltd.
Figures
References
-
- Greenblatt DJ, Harmatz JS, von Moltke LL, et al. Comparative kinetics, and dynamics of zaleplon, zolpidem, and placebo. Clin Pharmacol Ther. 1998;64:553–561. - PubMed
-
- Hohagen F, Kappler C, Schramm E, et al. Prevalence of insomnia in elderly general practice attenders and the current treatment modalities. Acta Psychiatr Scand. 1994a;902:102–108. - PubMed
-
- Hohagen F, Kappler C, Schramm E, et al. Sleep onset insomnia; sleep maintaining insomnia and insomnia with early morning awakening—temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994b;17:551–554. - PubMed
-
- Kaplan GB, Greenblatt DJ, Ehrenberg BL, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37:693–703. - PubMed
-
- Mellinger G, Balter M, Uhlenhuth E. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatr. 1985;42:225–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources