Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping
- PMID: 17907801
- PMCID: PMC2323294
- DOI: 10.1371/journal.ppat.0030130
Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping
Abstract
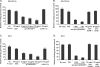
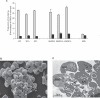
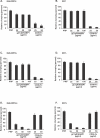
The ability of Plasmodium falciparum-infected red blood cells (IRBCs) to bind to vascular endothelium, thus enabling sequestration in vital host organs, is an important pathogenic mechanism in malaria. Adhesion of P. falciparum IRBCs to platelets, which results in the formation of IRBC clumps, is another cytoadherence phenomenon that is associated with severe disease. Here, we have used in vitro cytoadherence assays to demonstrate, to our knowledge for the first time, that P. falciparum IRBCs use the 32-kDa human protein gC1qR/HABP1/p32 as a receptor to bind to human brain microvascular endothelial cells. In addition, we show that P. falciparum IRBCs can also bind to gC1qR/HABP1/p32 on platelets to form clumps. Our study has thus identified a novel host receptor that is used for both adhesion to vascular endothelium and platelet-mediated clumping. Given the association of adhesion to vascular endothelium and platelet-mediated clumping with severe disease, adhesion to gC1qR/HABP1/p32 by P. falciparum IRBCs may play an important role in malaria pathogenesis.
Conflict of interest statement
Figures
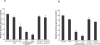
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

