TRAIL in cancer therapy: present and future challenges
- PMID: 17907960
- PMCID: PMC2976473
- DOI: 10.1517/14728222.11.10.1299
TRAIL in cancer therapy: present and future challenges
Abstract
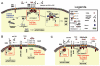
Since its identification in 1995, TNF-related apoptosis-inducing ligand (TRAIL) has sparked growing interest in oncology due to its reported ability to selectively trigger cancer cell death. In contrast to other members of the TNF superfamily, TRAIL administration in vivo is safe. The relative absence of toxic side effects of this naturally occurring cytokine, in addition to its antitumoural properties, has led to its preclinical evaluation. However, despite intensive investigations, little is known in regards to the mechanisms underlying TRAIL selectivity or efficiency. An appropriate understanding of its physiological relevance, and of the mechanisms controlling cancer cells escape from TRAIL-induced cell death, will be required to optimally use the cytokine in clinics. The present review focuses on recent advances in the understanding of TRAIL signal transduction and discusses the existing and future challenges of TRAIL-based cancer therapy development.
Figures
References
-
- PITTI RM, MARSTERS SA, RUPPERT S, et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271(22):12687–12690. - PubMed
-
- WILEY SR, SCHOOLEY K, SMOLAK PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. - PubMed
-
- PAN G, O’ROURKE K, CHINNAIYAN AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276(5309):111–113. - PubMed
-
- CHAUDHARY PM, EBY M, JASMIN A, et al. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7(6):821–830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
