The regulation of M1 muscarinic acetylcholine receptor desensitization by synaptic activity in cultured hippocampal neurons
- PMID: 17908240
- PMCID: PMC2658029
- DOI: 10.1111/j.1471-4159.2007.04931.x
The regulation of M1 muscarinic acetylcholine receptor desensitization by synaptic activity in cultured hippocampal neurons
Abstract
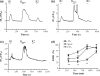
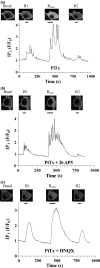
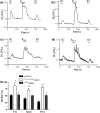
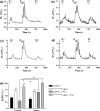
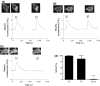
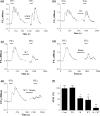
To better understand metabotropic/ionotropic integration in neurons we have examined the regulation of M1 muscarinic acetylcholine (mACh) receptor signalling in mature (> 14 days in vitro), synaptically-active hippocampal neurons in culture. Using a protocol where neurons are exposed to an EC(50) concentration of the muscarinic agonist methacholine (MCh) prior to (R1), and following (R2) a desensitizing pulse of a high concentration of this agonist, we have found that the reduction in M(1) mACh receptor responsiveness is decreased in quiescent (+tetrodotoxin) neurons and increased when synaptic activity is enhanced by blocking GABA(A) receptors with picrotoxin. The picrotoxin-mediated effect on M1 mACh receptor responsiveness was completely prevented by alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor blockade. Inhibition of endogenous G protein-coupled receptor kinase 2 by transfection with the non-G(q/11)alpha-binding, catalytically-inactive (D110A,K220R)G protein-coupled receptor kinase 2 mutant, decreased the extent of M1 mACh receptor desensitization under all conditions. Pharmacological inhibition of protein kinase C (PKC) activity, or chronic phorbol ester-induced PKC down-regulation had no effect on agonist-mediated receptor desensitization in quiescent or spontaneously synaptically active neurons, but significantly decreased the extent of receptor desensitization in picrotoxin-treated neurons. MCh stimulated the translocation of diacylglycerol- sensitive eGFP-PKCepsilon, but not Ca2+/diacylglycerol-sensitive eGFP-PKCbetaII in both the absence, and presence of tetrodotoxin. Under these conditions, MCh-stimulated eGFP-myristoylated, alanine-rich C kinase substrate translocation was dependent on PKC activity, but not Ca2+/calmodulin. In contrast, picrotoxin-driven translocation of myristoylated, alanine-rich C kinase substrate was accompanied by translocation of PKCbetaII, but not PKCepsilon, and was dependent on PKC and Ca2+/calmodulin. Taken together these data suggest that the level of synaptic activity may determine the different kinases recruited to regulate M1 mACh receptor desensitization in neurons.
Figures


Similar articles
-
Roles of phosphorylation-dependent and -independent mechanisms in the regulation of M1 muscarinic acetylcholine receptors by G protein-coupled receptor kinase 2 in hippocampal neurons.J Biol Chem. 2005 May 13;280(19):18950-8. doi: 10.1074/jbc.M412682200. Epub 2005 Mar 2. J Biol Chem. 2005. PMID: 15743771
-
Imaging of muscarinic acetylcholine receptor signaling in hippocampal neurons: evidence for phosphorylation-dependent and -independent regulation by G-protein-coupled receptor kinases.J Neurosci. 2004 Apr 28;24(17):4157-62. doi: 10.1523/JNEUROSCI.5506-03.2004. J Neurosci. 2004. PMID: 15115810 Free PMC article.
-
NMDA-receptor regulation of muscarinic-receptor stimulated inositol 1,4,5-trisphosphate production and protein kinase C activation in single cerebellar granule neurons.J Neurochem. 2004 Jun;89(6):1537-46. doi: 10.1111/j.1471-4159.2004.02458.x. J Neurochem. 2004. PMID: 15189357
-
Muscarinic M1 acetylcholine receptors regulate the non-quantal release of acetylcholine in the rat neuromuscular junction via NO-dependent mechanism.J Neurochem. 2007 Sep;102(6):2110-2117. doi: 10.1111/j.1471-4159.2007.04696.x. Epub 2007 Jun 11. J Neurochem. 2007. PMID: 17561934
-
Regulation of Src family kinases by muscarinic acetylcholine receptors in heterologous cells and neurons.Front Mol Neurosci. 2024 Jan 11;16:1340725. doi: 10.3389/fnmol.2023.1340725. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38273940 Free PMC article. Review.
Cited by
-
Nicotinic and muscarinic acetylcholine receptors shape ganglion cell response properties.J Neurophysiol. 2015 Jan 1;113(1):203-17. doi: 10.1152/jn.00405.2014. Epub 2014 Oct 8. J Neurophysiol. 2015. PMID: 25298382 Free PMC article.
-
Reduced expression of G protein-coupled receptor kinases in schizophrenia but not in schizoaffective disorder.Neurobiol Dis. 2011 Nov;44(2):248-58. doi: 10.1016/j.nbd.2011.07.009. Epub 2011 Jul 20. Neurobiol Dis. 2011. PMID: 21784156 Free PMC article.
-
Receptor Species-dependent Desensitization Controls KCNQ1/KCNE1 K+ Channels as Downstream Effectors of Gq Protein-coupled Receptors.J Biol Chem. 2016 Dec 16;291(51):26410-26426. doi: 10.1074/jbc.M116.746974. Epub 2016 Nov 10. J Biol Chem. 2016. PMID: 27834678 Free PMC article.
-
Temporal profiling of changes in phosphatidylinositol 4,5-bisphosphate, inositol 1,4,5-trisphosphate and diacylglycerol allows comprehensive analysis of phospholipase C-initiated signalling in single neurons.J Neurochem. 2008 Nov;107(3):602-15. doi: 10.1111/j.1471-4159.2008.05587.x. Epub 2008 Aug 11. J Neurochem. 2008. PMID: 18665913 Free PMC article.
-
Optogenetic reporters: Fluorescent protein-based genetically encoded indicators of signaling and metabolism in the brain.Prog Brain Res. 2012;196:235-63. doi: 10.1016/B978-0-444-59426-6.00012-4. Prog Brain Res. 2012. PMID: 22341329 Free PMC article. Review.
References
-
- Bacci A, Verderio C, Pravettoni E, Matteoli M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur. J. Neurosci. 1999;11:389–397. - PubMed
-
- Bartlett P J, Young K W, Nahorski S R, Challiss R A J. Single cell analysis and temporal profiling of agonist-mediated inositol 1,4,5-trisphosphate, Ca2+, diacylglycerol, and protein kinase C signaling using fluorescent biosensors. J. Biol. Chem. 2005;280:21837–21846. - PubMed
-
- Berkeley J L, Levey A I. Cell-specific extracellular signal-regulated kinase activation by multiple G protein-coupled receptor families in hippocampus. Mol. Pharmacol. 2003;63:128–135. - PubMed
-
- Berkeley J L, Gomeza J, Wess J, Hamilton S E, Nathanson N M, Levey A I. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol. Cell. Neurosci. 2001;18:512–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

