Paradoxical antiproliferative effect by a murine mammary tumor-derived epithelial cell line
- PMID: 17908302
- PMCID: PMC2129098
- DOI: 10.1186/1471-2407-7-184
Paradoxical antiproliferative effect by a murine mammary tumor-derived epithelial cell line
Abstract
Background: Despite significant advancement in breast cancer therapy, there is a great need for a better understanding of the mechanisms involved in breast carcinogenesis and progression, as well as of the role of epigenetic contributions from stromal cells in mammary tumorigenesis. In this study, we isolated and characterized murine mammary tumor-derived epithelial and myofibroblast cell lines, and investigated the in vitro and in vivo effect of cellular soluble factors produced by the epithelial cell line on tumor cells.
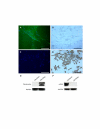
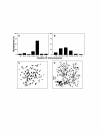
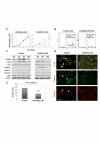
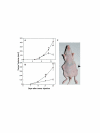
Methods: Morphology, immunophenotype, cytogenetics, invasiveness, and tumorigenicity of epithelial (LM-234ep) and myofibroblast (LM-234mf) cell lines isolated from two murine mammary adenocarcinomas with common ancestor were studied. The in vitro effects of LM-234ep conditioned medium on proliferation, cell cycle distribution, and expression of cell cycle proteins, were investigated in LM-234mf cells, mouse melanoma cells (B16-F10), and human cervical adenocarcinoma cells (HeLa). The in vivo anti-tumor activity of LM-234ep conditioned media was evaluated in subcutaneous tumors formed in nude mice by B16-F10 and HeLa cells.
Results: LM-234ep cells were found to be cytokeratin positive and hipertriploid, whereas LM-234mf cells were alpha-smooth muscle actin positive and hypohexaploid. Chromosome aberrations were found in both cases. Only LM-234mf revealed to be invasive in vitro and to secrete active MMP-2, though neither of the cell types were able to produce progressing tumors. LM-234ep-derived factors were able to inhibit the in vitro growth of LM-234mf, B16-F10, and HeLa cells, inducing cell cycle arrest in G0/G1 phase. The administration of LM-234ep conditioned medium inhibited the growth of B16-F10 and HeLa tumors in nude mice.
Conclusion: Our data suggest the existence of epithelial cell variants with tumor suppressive properties within mammary tumors. To our knowledge, this is the first report showing antiproliferative and antineoplastic activities induced by tumor-derived epithelial cells.
Figures




Similar articles
-
A human prostatic stromal myofibroblast cell line WPMY-1: a model for stromal-epithelial interactions in prostatic neoplasia.Carcinogenesis. 1999 Jul;20(7):1185-92. doi: 10.1093/carcin/20.7.1185. Carcinogenesis. 1999. PMID: 10383888
-
Epithelial and fibroblast cell lines derived from a spontaneous mammary carcinoma in a MMTV/neu transgenic mouse.In Vitro Cell Dev Biol Anim. 2002 Jun;38(6):326-33. doi: 10.1290/1071-2690(2002)038<0326:EAFCLD>2.0.CO;2. In Vitro Cell Dev Biol Anim. 2002. PMID: 12513120
-
Progression of mouse mammary tumors: MCP-1-TNFalpha cross-regulatory pathway and clonal expression of promalignancy and antimalignancy factors.Int J Cancer. 2003 Oct 10;106(6):879-86. doi: 10.1002/ijc.11337. Int J Cancer. 2003. PMID: 12918065
-
Ilexgenin A induces B16-F10 melanoma cell G1/S arrest in vitro and reduces tumor growth in vivo.Int Immunopharmacol. 2015 Feb;24(2):423-431. doi: 10.1016/j.intimp.2014.12.040. Epub 2015 Jan 13. Int Immunopharmacol. 2015. PMID: 25596038
-
Studies on mammary carcinogenesis induced by a heterocyclic amine, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, in mice and rats.Environ Mol Mutagen. 2002;39(2-3):158-64. doi: 10.1002/em.10047. Environ Mol Mutagen. 2002. PMID: 11921184 Review.
Cited by
-
Impact of Heat Stress on Cellular and Transcriptional Adaptation of Mammary Epithelial Cells in Riverine Buffalo (Bubalus Bubalis).PLoS One. 2016 Sep 28;11(9):e0157237. doi: 10.1371/journal.pone.0157237. eCollection 2016. PLoS One. 2016. PMID: 27682256 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

