Domain architecture of the catalytic subunit in the ISW2-nucleosome complex
- PMID: 17908792
- PMCID: PMC2169183
- DOI: 10.1128/MCB.01351-07
Domain architecture of the catalytic subunit in the ISW2-nucleosome complex
Abstract
ATP-dependent chromatin remodeling has an important role in the regulation of cellular differentiation and development. For the first time, a topological view of one of these complexes has been revealed, by mapping the interactions of the catalytic subunit Isw2 with nucleosomal and extranucleosomal DNA in the complex with all four subunits of ISW2 bound to nucleosomes. Different domains of Isw2 were shown to interact with the nucleosome near the dyad axis, another near the entry site of the nucleosome, and another with extranucleosomal DNA. The conserved DEXD or ATPase domain was found to contact the superhelical location 2 (SHL2) of the nucleosome, providing a direct physical connection of ATP hydrolysis with this region of nucleosomes. The C terminus of Isw2, comprising the SLIDE (SANT-like domain) and HAND domains, was found to be associated with extranucleosomal DNA and the entry site of nucleosomes. It is thus proposed that the C-terminal domains of Isw2 are involved in anchoring the complex to nucleosomes through their interactions with linker DNA and that they facilitate the movement of DNA along the surface of nucleosomes.
Figures
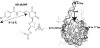



References
-
- Boyer, L. A., M. R. Langer, K. A. Crowley, S. Tan, J. M. Denu, and C. L. Peterson. 2002. Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol. Cell 10:935-942. - PubMed
-
- Cote, J., J. Quinn, J. L. Workman, and C. L. Peterson. 1994. Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex. Science 265:53-60. - PubMed
-
- Dang, W., M. N. Kagalwala, and B. Bartholomew. 2007. The Dpb4 subunit of ISW2 is anchored to extranucleosomal DNA. J. Biol. Chem. 282:19418-19425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
