Hybrid cell vaccination resolves Leishmania donovani infection by eliciting a strong CD8+ cytotoxic T-lymphocyte response with concomitant suppression of interleukin-10 (IL-10) but not IL-4 or IL-13
- PMID: 17908806
- PMCID: PMC2168357
- DOI: 10.1128/IAI.00944-07
Hybrid cell vaccination resolves Leishmania donovani infection by eliciting a strong CD8+ cytotoxic T-lymphocyte response with concomitant suppression of interleukin-10 (IL-10) but not IL-4 or IL-13
Abstract
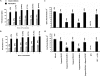
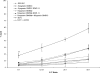
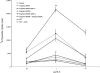
There is an acute dearth of therapeutic interventions against visceral leishmaniasis that is required to restore an established defective cell-mediated immune response. Hence, formulation of effective immunotherapy requires the use of dominant antigen(s) targeted to elicit a specific antiparasitic cellular immune response. We implemented hybrid cell vaccination therapy in Leishmania donovani-infected BALB/c mice by electrofusing dominant Leishmania antigen kinetoplastid membrane protein 11 (KMP-11)-transfected bone marrow-derived macrophages from BALB/c mice with allogeneic bone marrow-derived dendritic cells from C57BL/6 mice. Hybrid cell vaccine (HCV) cleared the splenic and hepatic parasite burden, eliciting KMP-11-specific major histocompatibility complex class I-restricted CD8+ cytotoxic T-lymphocyte (CTL) responses. Moreover, splenic lymphocytes of HCV-treated mice not only showed the enhancement of gamma interferon but also marked an elevated expression of the Th2 cytokines interleukin-4 (IL-4) and IL-13 at both transcriptional and translational levels. On the other hand, IL-10 production from splenic T cells was markedly suppressed as a result of HCV therapy. CD8+ T-cell depletion completely abrogated HCV-mediated immunity and the anti-KMP-11 CTL response. Interestingly, CD8+ T-cell depletion completely abrogated HCV-induced immunity, resulting in a marked increase of IL-10 but not of IL-4 and IL-13. The present study reports the first implementation of HCV immunotherapy in an infectious disease model, establishing strong antigen-specific CTL generation as a correlate of HCV-mediated antileishmanial immunity that is reversed by in vivo CD8+ T-cell depletion of HCV-treated mice. Our findings might be extended to drug-nonresponsive visceral leishmaniasis patients, as well as against multiple infectious diseases with pathogen-specific immunodominant antigens.
Figures

References
-
- Ahuja, S. S., R. L. Reddick, N. Sato, E. Montalbo, V. Kostecki, W. Zhao, M. J. Dolan, P. C. Melby, and S. K. Ahuja. 1999. Dendritic cell (DC)-based anti-infective strategies: DCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J. Immunol. 163:3890-3897. - PubMed
-
- Alexander, J., K. C. Carter, N. Al-Fasi, A. Satoskar, and F. Brombacher. 2000. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur. J. Immunol. 30:2935-2943. - PubMed
-
- Avigan, D., B. Vasir, J. Gong, V. Borges, Z. Wu, L. Uhl, M. Atkins, J. Mier, D. McDermott, T. Smith, N. Giallambardo, C. Stone, K. Schadt, J. Dolgoff, J. C. Tetreault, M. Villarroel, and D. Kufe. 2004. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin. Cancer Res. 10:4699-4708. - PubMed
-
- Basak, S. K., B. Saha, A. Bhattacharya, and S. Roy. 1992. Immunobiological studies on experimental visceral leishmaniasis. II. Adherent cell-mediated down-regulation of delayed-type hypersensitivity response and up-regulation of B-cell activation. Eur. J. Immunol. 22:2041-2045. - PubMed
-
- Basu, R., S. Roy, and P. Walden. 2007. HLA class I-restricted T-cell epitopes of the kinetoplastid membrane protein presented by Leishmania donovani-infected human macrophages. J. Infect. Dis. 195:1373-1380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

