Combination of protein and viral vaccines induces potent cellular and humoral immune responses and enhanced protection from murine malaria challenge
- PMID: 17908809
- PMCID: PMC2168343
- DOI: 10.1128/IAI.00828-07
Combination of protein and viral vaccines induces potent cellular and humoral immune responses and enhanced protection from murine malaria challenge
Abstract
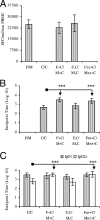
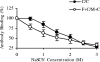
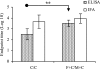
The search for an efficacious vaccine against malaria is ongoing, and it is now widely believed that to confer protection a vaccine must induce very strong cellular and humoral immunity concurrently. We studied the immune response in mice immunized with the recombinant viral vaccines fowlpox strain FP9 and modified virus Ankara (MVA), a protein vaccine (CV-1866), or a combination of the two; all vaccines express parts of the same preerythrocytic malaria antigen, the Plasmodium berghei circumsporozoite protein (CSP). Mice were then challenged with P. berghei sporozoites to determine the protective efficacies of different vaccine regimens. Two immunizations with the protein vaccine CV-1866, based on the hepatitis B core antigen particle, induced strong humoral immunity to the repeat region of CSP that was weakly protective against sporozoite challenge. Prime-boost with the viral vector vaccines, FP9 followed by MVA, induced strong T-cell immunity to the CD8+ epitope Pb9 and partially protected animals from challenge. Physically mixing CV-1866 with FP9 or MVA and then immunizing with the resultant combinations in a prime-boost regimen induced both cellular and humoral immunity and afforded substantially higher levels of protection (combination, 90%) than either vaccine alone (CV-1866, 12%; FP9/MVA, 37%). For diseases such as malaria in which different potent immune responses are required to protect against different stages, using combinations of partially effective vaccines may offer a more rapid route to achieving deployable levels of efficacy than individual vaccine strategies.
Figures
Similar articles
-
Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus.J Immunol. 2004 Mar 1;172(5):3094-100. doi: 10.4049/jimmunol.172.5.3094. J Immunol. 2004. PMID: 14978115
-
Importance of the Immunodominant CD8+ T Cell Epitope of Plasmodium berghei Circumsporozoite Protein in Parasite- and Vaccine-Induced Protection.Infect Immun. 2020 Sep 18;88(10):e00383-20. doi: 10.1128/IAI.00383-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32719159 Free PMC article.
-
Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine.Malar J. 2013 Apr 22;12:136. doi: 10.1186/1475-2875-12-136. Malar J. 2013. PMID: 23607541 Free PMC article.
-
Progress in DNA-based heterologous prime-boost immunization strategies for malaria.Immunol Rev. 2004 Jun;199:126-43. doi: 10.1111/j.0105-2896.2004.00138.x. Immunol Rev. 2004. PMID: 15233731 Review.
-
Prime-boost vectored malaria vaccines: progress and prospects.Hum Vaccin. 2010 Jan;6(1):78-83. doi: 10.4161/hv.6.1.10116. Epub 2010 Jan 18. Hum Vaccin. 2010. PMID: 20061802 Review.
Cited by
-
Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis.Sci Rep. 2013 Dec 19;3:3549. doi: 10.1038/srep03549. Sci Rep. 2013. PMID: 24351974 Free PMC article.
-
Co-expression of Interleukin-15 Enhances the Protective Immune Responses Induced by Immunization with a Murine Malaria MVA-Based Vaccine Encoding the Circumsporozoite Protein.PLoS One. 2015 Oct 27;10(10):e0141141. doi: 10.1371/journal.pone.0141141. eCollection 2015. PLoS One. 2015. PMID: 26505634 Free PMC article.
-
The march toward malaria vaccines.Vaccine. 2015 Nov 27;33 Suppl 4(Suppl 4):D13-23. doi: 10.1016/j.vaccine.2015.07.091. Epub 2015 Aug 29. Vaccine. 2015. PMID: 26324116 Free PMC article. Review.
-
The March Toward Malaria Vaccines.Am J Prev Med. 2015 Dec;49(6 Suppl 4):S319-33. doi: 10.1016/j.amepre.2015.09.011. Am J Prev Med. 2015. PMID: 26590432 Free PMC article. Review.
-
Recombinant peptide replicates immunogenicity of synthetic linear peptide chimera for use as pre-erythrocytic stage malaria vaccine.Microbes Infect. 2009 Jan;11(1):83-91. doi: 10.1016/j.micinf.2008.10.009. Epub 2008 Oct 26. Microbes Infect. 2009. PMID: 19015042 Free PMC article.
References
-
- Akanmori, B. D., S. Waki, and M. Suzuki. 1994. Immunoglobulin G2a isotype may have a protective role in Plasmodium berghei NK65 infection in immunised mice. Parasitol. Res. 80:638-641. - PubMed
-
- Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. - PubMed
-
- Anderson, R. J., C. M. Hannan, S. C. Gilbert, S. M. Laidlaw, E. G. Sheu, S. Korten, R. Sinden, G. A. Butcher, M. A. Skinner, and A. V. Hill. 2004. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J. Immunol. 172:3094-3100. - PubMed
-
- Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. - PubMed
-
- Birkett, A., K. Lyons, A. Schmidt, D. Boyd, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. Nardin. 2002. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infect. Immun. 70:6860-6870. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

