Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B
- PMID: 17908810
- PMCID: PMC2168345
- DOI: 10.1128/IAI.00846-07
Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B
Abstract
The bacterium Neisseria meningitidis is the causative agent of meningitis and sepsis. A generally effective vaccine against N. meningitidis serogroup B is not yet available, but outer membrane vesicle vaccines are in development. These vaccines contain lipopolysaccharide (LPS). The inclusion of N. meningitidis wild-type LPS in a vaccine is controversial because of its high toxicity. Therefore, the adjuvant activity of a panel of different Toll-like receptor (TLR) agonists in combination with LPS-deficient meningococcal outer membrane complexes was compared after immunization of mice. The results demonstrate that TLR3, TLR4, TLR7, and TLR9 agonists enhance immune responses against LPS-deficient outer membrane complexes. Their adjuvant activity was characterized by higher levels of antigen-specific immunoglobulin G (IgG), IgG2a, and IgG2b; a higher IgG2a/IgG1 ratio; lower total IgE levels; and most importantly, higher serum bactericidal antibody titers compared to LPS-deficient outer membrane complexes alone.
Figures
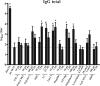
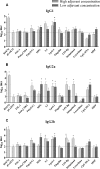
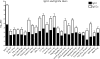
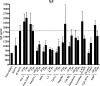
Similar articles
-
New proteoliposome vaccine formulation from N. meningitidis serogroup B, without aluminum hydroxide, retains its antimeningococcal protectogenic potential as well as Th-1 adjuvant capacity.BMC Immunol. 2013;14 Suppl 1(Suppl 1):S12. doi: 10.1186/1471-2172-14-S1-S12. Epub 2013 Feb 25. BMC Immunol. 2013. PMID: 23458443 Free PMC article.
-
Immunogenicity of outer membrane proteins in a lipopolysaccharide-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response.Infect Immun. 1999 Oct;67(10):4988-93. doi: 10.1128/IAI.67.10.4988-4993.1999. Infect Immun. 1999. PMID: 10496868 Free PMC article.
-
Evaluation of immunological responses to recombinant Porin A protein (rPoA) from native strains of Neisseria meningitidis serogroups A and B using OMV as an adjuvant in BALB/c mice.Microb Pathog. 2017 Nov;112:209-214. doi: 10.1016/j.micpath.2017.09.038. Epub 2017 Sep 20. Microb Pathog. 2017. PMID: 28942175
-
Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines.Expert Rev Vaccines. 2011 Mar;10(3):323-34. doi: 10.1586/erv.11.10. Expert Rev Vaccines. 2011. PMID: 21434800 Review.
-
A combination recombinant protein and outer membrane vesicle vaccine against serogroup B meningococcal disease.Expert Rev Vaccines. 2011 May;10(5):575-88. doi: 10.1586/erv.11.32. Expert Rev Vaccines. 2011. PMID: 21604979 Review.
Cited by
-
Delineating the Plausible Molecular Vaccine Candidates and Drug Targets of Multidrug-Resistant Acinetobacter baumannii.Front Cell Infect Microbiol. 2019 Jun 20;9:203. doi: 10.3389/fcimb.2019.00203. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31281799 Free PMC article.
-
Adjuvant effects elicited by novel oligosaccharide variants of detoxified meningococcal lipopolysaccharides on Neisseria meningitidis recombinant PorA protein: a comparison in mice.PLoS One. 2014 Dec 29;9(12):e115713. doi: 10.1371/journal.pone.0115713. eCollection 2014. PLoS One. 2014. PMID: 25545241 Free PMC article.
-
OMV Vaccines and the Role of TLR Agonists in Immune Response.Int J Mol Sci. 2020 Jun 21;21(12):4416. doi: 10.3390/ijms21124416. Int J Mol Sci. 2020. PMID: 32575921 Free PMC article. Review.
-
Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii.Infect Immun. 2011 Jan;79(1):518-26. doi: 10.1128/IAI.00741-10. Epub 2010 Oct 25. Infect Immun. 2011. PMID: 20974823 Free PMC article.
-
Bacteria derived extracellular vesicles in the pathogenesis and treatment of gastrointestinal tumours.Front Oncol. 2023 Jan 23;12:1103446. doi: 10.3389/fonc.2022.1103446. eCollection 2022. Front Oncol. 2023. PMID: 36776356 Free PMC article. Review.
References
-
- Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
-
- Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. - PubMed
-
- Blander, J. M., and R. Medzhitov. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808-812. - PubMed
-
- Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y. J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 197:101-109. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

