Phosphoinositide-3-kinase-dependent, MyD88-independent induction of CC-type chemokines characterizes the macrophage response to Toxoplasma gondii strains with high virulence
- PMID: 17908814
- PMCID: PMC2168350
- DOI: 10.1128/IAI.00821-07
Phosphoinositide-3-kinase-dependent, MyD88-independent induction of CC-type chemokines characterizes the macrophage response to Toxoplasma gondii strains with high virulence
Abstract
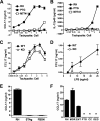
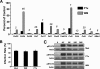
Chemokines play an important role in inflammation and infection due to their ability to recruit cells of innate and adaptive immunity. Here we examined mouse macrophage chemokine responses during intracellular infections with high- and low-virulence Toxoplasma gondii strains. The high-virulence type I strain RH induced a large panel of CC-type chemokines, whereas responses elicited by strains PTG (type II) and M7741 (type III) were much weaker. Strikingly, the T. gondii-induced chemokine response occurred independently of signaling through the Toll-like receptor adaptor MyD88. Instead, production of chemokines during infection was heavily dependent upon phosphoinositide-3-kinase signaling pathways. Because infection with type I strains such as RH results in an uncontrolled proinflammatory cytokine response, we hypothesize that this virulence phenotype is a consequence of early strong induction of chemokines by type I, but not type II or III, Toxoplasma strains.
Figures




References
-
- Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnage, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat. Immunol. 1:83-87. - PubMed
-
- Aliberti, J., J. G. Valenzuela, V. B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J. M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485-490. - PubMed
-
- Allen, S. J., S. E. Crown, and T. M. Handel. 2007. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 25:787-820. - PubMed
-
- Bjorkbacka, H., K. A. Fitzgerald, F. Huet, X. Li, J. A. Gregory, M. A. Lee, C. M. Ordija, N. E. Dowley, D. T. Golenbock, and M. W. Freeman. 2004. The induction of macrophage gene expression by LPS predominantly utilizes MyD88-independent signaling cascades. Physiol. Genomics 19:319-330. - PubMed
-
- Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

