Reductive evolution of architectural repertoires in proteomes and the birth of the tripartite world
- PMID: 17908824
- PMCID: PMC2045140
- DOI: 10.1101/gr.6454307
Reductive evolution of architectural repertoires in proteomes and the birth of the tripartite world
Abstract
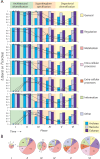
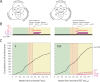
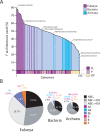
The repertoire of protein architectures in proteomes is evolutionarily conserved and capable of preserving an accurate record of genomic history. Here we use a census of protein architecture in 185 genomes that have been fully sequenced to generate genome-based phylogenies that describe the evolution of the protein world at fold (F) and fold superfamily (FSF) levels. The patterns of representation of F and FSF architectures over evolutionary history suggest three epochs in the evolution of the protein world: (1) architectural diversification, where members of an architecturally rich ancestral community diversified their protein repertoire; (2) superkingdom specification, where superkingdoms Archaea, Bacteria, and Eukarya were specified; and (3) organismal diversification, where F and FSF specific to relatively small sets of organisms appeared as the result of diversification of organismal lineages. Functional annotation of FSF along these architectural chronologies revealed patterns of discovery of biological function. Most importantly, the analysis identified an early and extensive differential loss of architectures occurring primarily in Archaea that segregates the archaeal lineage from the ancient community of organisms and establishes the first organismal divide. Reconstruction of phylogenomic trees of proteomes reflects the timeline of architectural diversification in the emerging lineages. Thus, Archaea undertook a minimalist strategy using only a small subset of the full architectural repertoire and then crystallized into a diversified superkingdom late in evolution. Our analysis also suggests a communal ancestor to all life that was molecularly complex and adopted genomic strategies currently present in Eukarya.
Figures



Similar articles
-
The evolutionary history of protein fold families and proteomes confirms that the archaeal ancestor is more ancient than the ancestors of other superkingdoms.BMC Evol Biol. 2012 Jan 27;12:13. doi: 10.1186/1471-2148-12-13. BMC Evol Biol. 2012. PMID: 22284070 Free PMC article.
-
Origin and evolution of protein fold designs inferred from phylogenomic analysis of CATH domain structures in proteomes.PLoS Comput Biol. 2013;9(3):e1003009. doi: 10.1371/journal.pcbi.1003009. Epub 2013 Mar 28. PLoS Comput Biol. 2013. PMID: 23555236 Free PMC article.
-
Global patterns of protein domain gain and loss in superkingdoms.PLoS Comput Biol. 2014 Jan 30;10(1):e1003452. doi: 10.1371/journal.pcbi.1003452. eCollection 2014 Jan. PLoS Comput Biol. 2014. PMID: 24499935 Free PMC article.
-
The origin, evolution and structure of the protein world.Biochem J. 2009 Feb 1;417(3):621-37. doi: 10.1042/BJ20082063. Biochem J. 2009. PMID: 19133840 Review.
-
Transfer RNA and the origins of diversified life.Sci Prog. 2008;91(Pt 3):265-84. doi: 10.3184/003685008X360650. Sci Prog. 2008. PMID: 18853577 Free PMC article. Review.
Cited by
-
The enzymatic and metabolic capabilities of early life.PLoS One. 2012;7(9):e39912. doi: 10.1371/journal.pone.0039912. Epub 2012 Sep 10. PLoS One. 2012. PMID: 22970111 Free PMC article.
-
Identification of functional signatures in the metabolism of the three cellular domains of life.PLoS One. 2019 May 28;14(5):e0217083. doi: 10.1371/journal.pone.0217083. eCollection 2019. PLoS One. 2019. PMID: 31136618 Free PMC article.
-
Effects of Structural Isomers of Spermine on the Higher-Order Structure of DNA and Gene Expression.Int J Mol Sci. 2021 Feb 26;22(5):2355. doi: 10.3390/ijms22052355. Int J Mol Sci. 2021. PMID: 33652986 Free PMC article.
-
The Unfinished Reconstructed Nature of the Last Universal Common Ancestor.J Mol Evol. 2024 Oct;92(5):584-592. doi: 10.1007/s00239-024-10187-8. Epub 2024 Jul 18. J Mol Evol. 2024. PMID: 39026043 Free PMC article. Review.
-
Comparative analysis of barophily-related amino acid content in protein domains of Pyrococcus abyssi and Pyrococcus furiosus.Archaea. 2013;2013:680436. doi: 10.1155/2013/680436. Epub 2013 Sep 26. Archaea. 2013. PMID: 24187517 Free PMC article.
References
-
- Ancel L.W., Fontana W., Fontana W. Plasticity, evolvability, and modularity in RNA. J. Exp. Zool. 2000;288:242–283. - PubMed
-
- Andreeva A., Howorth D., Brenner S.E., Hubbard T.J.P., Chothia C., Murzin A.G., Howorth D., Brenner S.E., Hubbard T.J.P., Chothia C., Murzin A.G., Brenner S.E., Hubbard T.J.P., Chothia C., Murzin A.G., Hubbard T.J.P., Chothia C., Murzin A.G., Chothia C., Murzin A.G., Murzin A.G. SCOP database in 2004: Refinements integrate structure and sequence family data. Nucleic Acids Res. 2004;32:D226–D229. doi: 10.1093/nar/gkh039. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
