Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p
- PMID: 17908928
- PMCID: PMC1993872
- DOI: 10.1101/gad.439307
Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p
Abstract
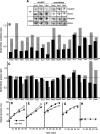
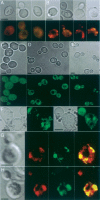
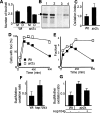
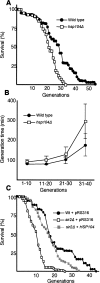
The levels of oxidatively damaged, carbonylated, proteins increase with the replicative age of yeast mother cells. We show here that such carbonylated proteins are associated with Hsp104p-containing protein aggregates and that these aggregates, like oxidized proteins, are retained in the progenitor cell during cytokinesis by a Sir2p-dependent process. Deletion of HSP104 resulted in a breakdown of damage asymmetry, and overproduction of Hsp104p partially restored damage retention in sir2Delta cells, suggesting that functional chaperones associated with protein aggregates are required for the establishment of damage asymmetry and that these functions are limited in sir2Delta cells. In line with this, Hsp104p and several Hsp70s displayed elevated damaged in sir2Delta cells, and protein aggregates were rescued at a slower rate in this mutant. Moreover, overproduction of Hsp104p suppressed the accelerated aging of cells lacking Sir2p, and drugs inhibiting damage segregation further demonstrated that spatial quality control is required to rejuvenate the progeny.
Figures




References
-
- Aguilaniu H., Gustafsson L., Rigoulet M., Nyström T., Gustafsson L., Rigoulet M., Nyström T., Rigoulet M., Nyström T., Nyström T. Protein oxidation in G0 cells of Saccharomyces cerevisiae depends on the state rather than rate of respiration and is enhanced in pos9 but not yap1 mutants. J. Biol. Chem. 2001;276:35396–35404. - PubMed
-
- Aguilaniu H., Gustafsson L., Rigoulet M., Nyström T., Gustafsson L., Rigoulet M., Nyström T., Rigoulet M., Nyström T., Nyström T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. - PubMed
-
- Bota D.A., Davies K.J., Davies K.J. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. - PubMed
-
- Davies K.J. Protein damage and degradation by oxygen radicals. I. General aspects. J. Biol. Chem. 1987;262:9895–9901. - PubMed
-
- Desnues B., Cuny C., Gregori G., Dukan S., Aguilaniu H., Nyström T., Cuny C., Gregori G., Dukan S., Aguilaniu H., Nyström T., Gregori G., Dukan S., Aguilaniu H., Nyström T., Dukan S., Aguilaniu H., Nyström T., Aguilaniu H., Nyström T., Nyström T. Differential oxidative damage and expression of stress defence regulons in culturable and non-culturable cells of Escherichia coli cells. EMBO Rep. 2003;4:400–405. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
