Autocrine glutamate signaling promotes glioma cell invasion
- PMID: 17909056
- PMCID: PMC2045073
- DOI: 10.1158/0008-5472.CAN-07-2034
Autocrine glutamate signaling promotes glioma cell invasion
Erratum in
- Cancer Res. 2007 Nov 1;67(21):10624
Abstract
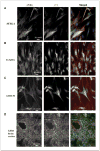
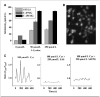
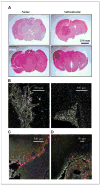
Malignant gliomas have been shown to release glutamate, which kills surrounding brain cells, creating room for tumor expansion. This glutamate release occurs primarily via system xC, a Na+-independent cystine-glutamate exchanger. We show here, in addition, that the released glutamate acts as an essential autocrine/paracrine signal that promotes cell invasion. Specifically, chemotactic invasion and scrape motility assays each show dose-dependent inhibition of cell migration when glutamate release was inhibited using either S-(4)-CPG or sulfasalazine, both potent blockers of system xC. This inhibition could be overcome by the addition of exogenous glutamate (100 micromol/L) in the continued presence of the inhibitors. Migration/invasion was also inhibited when Ca2+-permeable alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPA-R) were blocked using GYKI or Joro spider toxin, whereas CNQX was ineffective. Ca2+ imaging experiments show that the released glutamate activates Ca2+-permeable AMPA-R and induces intracellular Ca2+ oscillations that are essential for cell migration. Importantly, glioma cells release glutamate in sufficient quantities to activate AMPA-Rs on themselves or neighboring cells, thus acting in an autocrine and/or paracrine fashion. System xC and the appropriate AMPA-R subunits are expressed in all glioma cell lines, patient-derived glioma cells, and acute patient biopsies investigated. Furthermore, animal studies in which human gliomas were xenographed into scid mice show that chronic inhibition of system xC-mediated glutamate release leads to smaller and less invasive tumors compared with saline-treated controls. These data suggest that glioma invasion is effectively disrupted by inhibiting an autocrine glutamate signaling loop with a clinically approved candidate drug, sulfasalazine, already in hand.
Figures



References
-
- Laerum OD, Bjerkvig R, Steinsvag S, de Ridder L. Invasiveness of primary brain tumors. Cancer Metastasis Rev. 1984;3:223–36. [review] [91 refs] - PubMed
-
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–5. - PubMed
-
- Kaba SE, Kyritsis AP. Recognition and management of gliomas. Drugs. 1997;53:235–44. - PubMed
-
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–91. - PubMed
-
- Ishiuchi S, Tsuzuki K, Yoshida Y, et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

