The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory
- PMID: 17909100
- PMCID: PMC2044557
- DOI: 10.1101/lm.678307
The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory
Abstract
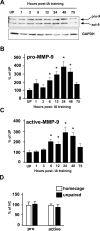
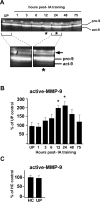
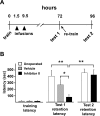
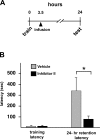
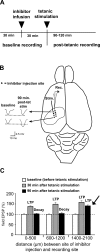
Matrix metalloproteinases (MMPs) are a family of extracellularly acting proteolytic enzymes with well-recognized roles in plasticity and remodeling of synaptic circuits during brain development and following brain injury. However, it is now becoming increasingly apparent that MMPs also function in normal, nonpathological synaptic plasticity of the kind that may underlie learning and memory. Here, we extend this idea by investigating the role and regulation of MMP-9 in an inhibitory avoidance (IA) learning and memory task. We demonstrate that following IA training, protein levels and proteolytic activity of MMP-9 become elevated in hippocampus by 6 h, peak at 12-24 h, then decline to baseline values by approximately 72 h. When MMP function is abrogated by intrahippocampal infusion of a potent gelatinase (MMP-2 and MMP-9) inhibitor 3.5 h following IA training, a time prior to the onset of training-induced elevation in levels, IA memory retention is significantly diminished when tested 1-3 d later. Animals impaired at 3 d exhibit robust IA memory when retrained, suggesting that such impairment is not likely attributed to toxic or other deleterious effects that permanently disrupt hippocampal function. In anesthetized adult rats, the effective distance over which synaptic plasticity is impaired by a single intrahippocampal infusion of the MMP inhibitor of the kind that blocks IA memory is approximately 1200 microm. Taken together, these data suggest that IA training induces a slowly emerging, but subsequently protracted period of MMP-mediated proteolysis critical for enabling long-lasting synaptic modification that underlies long-term memory consolidation.
Figures
Similar articles
-
In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity.J Neurophysiol. 2007 Jul;98(1):334-44. doi: 10.1152/jn.00202.2007. Epub 2007 May 9. J Neurophysiol. 2007. PMID: 17493927 Free PMC article.
-
Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory.J Neurosci. 2006 Feb 15;26(7):1923-34. doi: 10.1523/JNEUROSCI.4359-05.2006. J Neurosci. 2006. PMID: 16481424 Free PMC article.
-
Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity.J Neurochem. 2006 Mar;96(5):1227-41. doi: 10.1111/j.1471-4159.2005.03565.x. Epub 2006 Feb 8. J Neurochem. 2006. PMID: 16464240
-
The molecular cascades of long-term potentiation underlie memory consolidation of one-trial avoidance in the CA1 region of the dorsal hippocampus, but not in the basolateral amygdala or the neocortex.Neurotox Res. 2008 Oct;14(2-3):273-94. doi: 10.1007/BF03033816. Neurotox Res. 2008. PMID: 19073432 Review.
-
Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures.Neurobiol Learn Mem. 1997 Nov;68(3):285-316. doi: 10.1006/nlme.1997.3799. Neurobiol Learn Mem. 1997. PMID: 9398590 Review.
Cited by
-
Synaptic circuit remodelling by matrix metalloproteinases in health and disease.Nat Rev Neurosci. 2012 Nov;13(11):743-57. doi: 10.1038/nrn3320. Epub 2012 Oct 10. Nat Rev Neurosci. 2012. PMID: 23047773 Free PMC article. Review.
-
Sleep and Memory Consolidation Dysfunction in Psychiatric Disorders: Evidence for the Involvement of Extracellular Matrix Molecules.Front Neurosci. 2021 May 14;15:646678. doi: 10.3389/fnins.2021.646678. eCollection 2021. Front Neurosci. 2021. PMID: 34054408 Free PMC article. Review.
-
The role of matrix metalloproteinase-9 in negative reinforcement learning and plasticity in alcohol dependence.Addict Biol. 2020 Mar;25(2):e12715. doi: 10.1111/adb.12715. Epub 2019 Jan 16. Addict Biol. 2020. PMID: 30648329 Free PMC article.
-
Dynamic regulation of the extracellular matrix in reward memory processes: a question of time.Front Cell Neurosci. 2023 Jun 16;17:1208974. doi: 10.3389/fncel.2023.1208974. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37396928 Free PMC article. Review.
-
Circadian Rhythms of Perineuronal Net Composition.eNeuro. 2020 Jul 31;7(4):ENEURO.0034-19.2020. doi: 10.1523/ENEURO.0034-19.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32719104 Free PMC article.
References
-
- Arai K., Lee S.R., Lo E.H. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–264. - PubMed
-
- Bernard-Trifilo J.A., Kramar E.A., Torp R., Lin C.Y., Pineda E.A., Lynch G., Gall C.M. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J. Neurochem. 2005;93:834–849. - PubMed
-
- Bevilaqua L.R., Medina J.H., Izquierdo I., Cammarota M. Memory consolidation induces N-methyl-D-aspartic acid-receptor- and Ca2+/calmodulin-dependent protein kinase II-dependent modifications in α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor properties. Neuroscience. 2005;136:397–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
