Distribution of NOS isoforms in a porcine endotoxin shock model
- PMID: 17909454
- PMCID: PMC3341620
- DOI: 10.1097/shk.0b013e3181598b77
Distribution of NOS isoforms in a porcine endotoxin shock model
Abstract
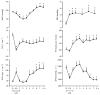
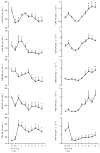
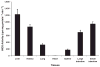
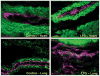
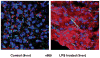
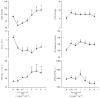
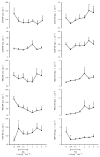
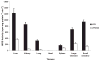
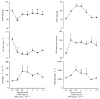
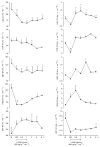
Sepsis is a major cause of morbidity and mortality. NO, an endogenous vasodilator, has been associated with the hypotension, catecholamine hyporesponsiveness, and myocardial depression of septic shock. Although iNOS is thought to be responsible for the hypotension and loss of vascular tone occurring several hours after endotoxin administration, little is known on the effects of constitutive eNOS on LPS-induced organ dysfunction. This study assessed the distribution of eNOS and iNOS in various vascular beds in conscious pigs challenged with LPS. Cardiac and regional hemodynamic parameters were recorded over 8 h in the presence and absence of aminoguanidine, a rather selective inhibitor of iNOS activity, and N-methyl-L-arginine, a nonspecific NOS inhibitor. Our data show that LPS-induced cardiac depression was associated with coronary, renal, and mesenteric vasoconstrictions and a hepatic vasodilatation. LPS also induced increases in eNOS in the heart and lungs, whereas iNOS was mostly detected in the liver. Nitrotyrosine formation was mainly detected in the lungs, with traces in the kidney, liver, and gut. Accordingly, our results suggest that the early decrease in blood pressure and cardiac depression are likely due to activated eNOS, whereas both isoforms are involved in the hepatic vasodilation. In contrast, carotid, coronary, mesenteric, and renal vasoconstrictions were significant at 5 and/ or 6 h after LPS infusion, suggesting that NO is not the primary mediator, facilitating and/or unmasking the release of vasoconstrictor mediators. Consequently, developing newer tissue- or isoform-specific NOS inhibitors can lead to novel therapeutic agents in septic shock.
Figures

Similar articles
-
Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock.Br J Pharmacol. 1995 Apr;114(8):1666-72. doi: 10.1111/j.1476-5381.1995.tb14955.x. Br J Pharmacol. 1995. PMID: 7541282 Free PMC article.
-
Pharmacological characterization of guanidinoethyldisulphide (GED), a novel inhibitor of nitric oxide synthase with selectivity towards the inducible isoform.Br J Pharmacol. 1996 Aug;118(7):1659-68. doi: 10.1111/j.1476-5381.1996.tb15589.x. Br J Pharmacol. 1996. PMID: 8842429 Free PMC article.
-
Effects of N-methyl-L-arginine on cardiac and regional blood flow in a dog endotoxin shock model.J Crit Care. 2000 Mar;15(1):22-9. doi: 10.1053/jcrc.2000.0150022. J Crit Care. 2000. PMID: 10757195
-
Involvement and dual effects of nitric oxide in septic shock.Inflamm Res. 1998 Apr;47(4):152-66. doi: 10.1007/s000110050309. Inflamm Res. 1998. PMID: 9628258 Review.
-
Nitric oxide and septic shock.Gen Pharmacol. 1997 Aug;29(2):159-66. doi: 10.1016/s0306-3623(96)00410-7. Gen Pharmacol. 1997. PMID: 9251894 Review.
Cited by
-
Are Temporal Differences in GDNF and NOS Isoform Induction Contributors to Neurodegeneration? A Fluorescence Microscopy-Based Study.Open Neurol J. 2016 Aug 26;10:67-76. doi: 10.2174/1874205X01610010067. eCollection 2016. Open Neurol J. 2016. PMID: 27651844 Free PMC article.
-
Cardiogenic shock and nutrition: safe?Intensive Care Med. 2011 Jan;37(1):35-45. doi: 10.1007/s00134-010-2061-8. Epub 2010 Nov 18. Intensive Care Med. 2011. PMID: 21086113 Review.
-
Mechanisms of Hemolysis During Sepsis.Inflammation. 2018 Oct;41(5):1569-1581. doi: 10.1007/s10753-018-0810-y. Inflammation. 2018. PMID: 29956069 Review.
-
Endothelial NOS (NOS3) impairs myocardial function in developing sepsis.Basic Res Cardiol. 2013 Mar;108(2):330. doi: 10.1007/s00395-013-0330-8. Epub 2013 Feb 10. Basic Res Cardiol. 2013. PMID: 23397596 Free PMC article.
-
Pathophysiological roles of peroxynitrite in circulatory shock.Shock. 2010 Sep;34 Suppl 1(0 1):4-14. doi: 10.1097/SHK.0b013e3181e7e9ba. Shock. 2010. PMID: 20523270 Free PMC article. Review.
References
-
- Kilbourn RG, Griffith OW. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992;84:827–831. - PubMed
-
- Julou-Schaeffer G, Gray GA, Fleming I, Schott C, Parratt JR, Stoclet JC. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990;259(4 Pt 2):H1038–H1043. - PubMed
-
- Klabunde RE, Ritger RC. NG-Monomethyl-L-arginine (NMA) restores arterial blood pressure but reduces cardiac output in a canine model of endotoxic shock. Biochem Biophys Res Commun. 1991;178:1135–1140. - PubMed
-
- Sharma VS, Traylor TG, Gardiner R, Mizukami H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry. 1987;26:3837–3843. - PubMed
-
- Kilbourn RG, Jubran A, Gross SS, Griffith OW, Levi R, Adams J, Lodato RF. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990;172:1132–1138. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

