Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay
- PMID: 17909504
- PMCID: PMC6149428
- DOI: 10.3390/12071496
Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay
Abstract
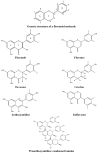
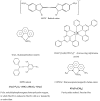
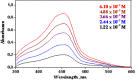
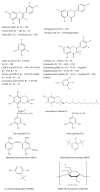
It would be desirable to establish and standardize methods that can measure the total antioxidant capacity level directly from vegetable extracts containing phenolics. Antioxidant capacity assays may be broadly classified as electron transfer (ET)- and hydrogen atom transfer (HAT)-based assays. The majority of HAT assays are kinetics-based, and involve a competitive reaction scheme in which antioxidant and substrate compete for peroxyl radicals thermally generated through the decomposition of azo compounds. ET-based assays measure the capacity of an antioxidant in the reduction of an oxidant, which changes colour when reduced. ET assays include the ABTS/TEAC, CUPRAC, DPPH, Folin-Ciocalteu and FRAP methods, each using different chromogenic redox reagents with different standard potentials. This review intends to offer a critical evaluation of existing antioxidant assays applied to phenolics, and reports the development by our research group of a simple and low-cost antioxidant capacity assay for dietary polyphenols, vitamins C and E, and human serum antioxidants, utilizing the copper(II)-neocuproine reagent as the chromogenic oxidizing agent, which we haved named the CUPRAC (cupric ion reducing antioxidant capacity) method. This method offers distinct advantages over other ET-based assays, namely the selection of working pH at physiological pH (as opposed to the Folin and FRAP methods, which work at alkaline and acidic pHs, respectively), applicability to both hydrophilic and lipophilic antioxidants (unlike Folin and DPPH), completion of the redox reactions for most common flavonoids (unlike FRAP), selective oxidation of antioxidant compounds without affecting sugars and citric acid commonly contained in foodstuffs and the capability to assay -SH bearing antioxidants (unlike FRAP). Other similar ET-based antioxidant assays that we have developed or modified for phenolics are the Fe(III)- and Ce(IV)-reducing capacity methods.
Figures


Similar articles
-
Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method.J Agric Food Chem. 2004 Dec 29;52(26):7970-81. doi: 10.1021/jf048741x. J Agric Food Chem. 2004. PMID: 15612784
-
Cupric ion reducing antioxidant capacity assay for food antioxidants: vitamins, polyphenolics, and flavonoids in food extracts.Methods Mol Biol. 2008;477:163-93. doi: 10.1007/978-1-60327-517-0_14. Methods Mol Biol. 2008. PMID: 19082947
-
Cupric ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers.Methods Mol Biol. 2010;594:215-39. doi: 10.1007/978-1-60761-411-1_15. Methods Mol Biol. 2010. PMID: 20072920
-
The chemistry behind antioxidant capacity assays.J Agric Food Chem. 2005 Mar 23;53(6):1841-56. doi: 10.1021/jf030723c. J Agric Food Chem. 2005. PMID: 15769103 Review.
-
Analytical Methods Used in Determining Antioxidant Activity: A Review.Int J Mol Sci. 2021 Mar 25;22(7):3380. doi: 10.3390/ijms22073380. Int J Mol Sci. 2021. PMID: 33806141 Free PMC article. Review.
Cited by
-
Evaluation of Phenolic Content Variability along with Antioxidant, Antimicrobial, and Cytotoxic Potential of Selected Traditional Medicinal Plants from India.Front Plant Sci. 2016 Mar 31;7:407. doi: 10.3389/fpls.2016.00407. eCollection 2016. Front Plant Sci. 2016. PMID: 27066046 Free PMC article.
-
Photoactive nanocatalysts as DTT-assisted BSA-AuNCs with enhanced oxidase-mimicking ability for sensitive fluorometric detection of antioxidants.J Nanobiotechnology. 2024 Sep 28;22(1):585. doi: 10.1186/s12951-024-02850-5. J Nanobiotechnology. 2024. PMID: 39342215 Free PMC article.
-
Berries and Leaves of Actinidia kolomikta (Rupr. & Maxim.) Maxim.: A Source of Phenolic Compounds.Plants (Basel). 2022 Jan 6;11(2):147. doi: 10.3390/plants11020147. Plants (Basel). 2022. PMID: 35050034 Free PMC article.
-
Simultaneously Determined Antioxidant and Pro-Oxidant Activity of Randomly Selected Plant Secondary Metabolites and Plant Extracts.Molecules. 2023 Sep 30;28(19):6890. doi: 10.3390/molecules28196890. Molecules. 2023. PMID: 37836733 Free PMC article.
-
Advances in the analytical methods for determining the antioxidant properties of honey: a review.Afr J Tradit Complement Altern Med. 2011 Oct 2;9(1):36-42. doi: 10.4314/ajtcam.v9i1.5. eCollection 2012. Afr J Tradit Complement Altern Med. 2011. PMID: 23983317 Free PMC article. Review.
References
-
- Robards K., Antolovich M. Analytical Chemistry of Fruit Bioflavonoids. A Review. Analyst. 1997;122:11–34. doi: 10.1039/a606499j. - DOI
-
- Balasundram N., Sundram K., Samman S. Phenolic Compounds in Plants and Agri-Industrial By-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. - DOI
-
- Harborne J.B., Simmonds N.W. Biochemistry of phenolic compounds. Academic Press; New York: 1964. The natural distribution of the phenolic aglycones; pp. 77–127.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

