CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration
- PMID: 17909628
- PMCID: PMC1994614
- DOI: 10.1172/JCI31692
CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration
Abstract
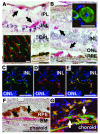
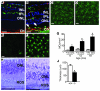
The role of retinal microglial cells (MCs) in age-related macular degeneration (AMD) is unclear. Here we demonstrated that all retinal MCs express CX3C chemokine receptor 1 (CX3CR1) and that homozygosity for the CX3CR1 M280 allele, which is associated with impaired cell migration, increases the risk of AMD. In humans with AMD, MCs accumulated in the subretinal space at sites of retinal degeneration and choroidal neovascularization (CNV). In CX3CR1-deficient mice, MCs accumulated subretinally with age and albino background and after laser impact preceding retinal degeneration. Raising the albino mice in the dark prevented both events. The appearance of lipid-bloated subretinal MCs was drusen-like on funduscopy of senescent mice, and CX3CR1-dependent MC accumulation was associated with an exacerbation of experimental CNV. These results show that CX3CR1-dependent accumulation of subretinal MCs evokes cardinal features of AMD. These findings reveal what we believe to be a novel pathogenic process with important implications for the development of new therapies for AMD.
Figures




Comment in
-
Overstaying their welcome: defective CX3CR1 microglia eyed in macular degeneration.J Clin Invest. 2007 Oct;117(10):2758-62. doi: 10.1172/JCI33513. J Clin Invest. 2007. PMID: 17909623 Free PMC article.
Similar articles
-
Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration.Invest Ophthalmol Vis Sci. 2007 Aug;48(8):3827-36. doi: 10.1167/iovs.07-0051. Invest Ophthalmol Vis Sci. 2007. PMID: 17652758 Free PMC article.
-
APOE Isoforms Control Pathogenic Subretinal Inflammation in Age-Related Macular Degeneration.J Neurosci. 2015 Oct 7;35(40):13568-76. doi: 10.1523/JNEUROSCI.2468-15.2015. J Neurosci. 2015. PMID: 26446211 Free PMC article.
-
Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice.Ophthalmic Res. 2008;40(3-4):115-9. doi: 10.1159/000119860. Epub 2008 Apr 18. Ophthalmic Res. 2008. PMID: 18421223 Free PMC article.
-
CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration.J Neuroinflammation. 2010 Dec 2;7:87. doi: 10.1186/1742-2094-7-87. J Neuroinflammation. 2010. PMID: 21126357 Free PMC article. Review.
-
Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration.Cells. 2020 May 14;9(5):1217. doi: 10.3390/cells9051217. Cells. 2020. PMID: 32423062 Free PMC article. Review.
Cited by
-
Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium.Invest Ophthalmol Vis Sci. 2015 May;56(5):3269-78. doi: 10.1167/iovs.15-16432. Invest Ophthalmol Vis Sci. 2015. PMID: 26024109 Free PMC article.
-
Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization.EMBO Mol Med. 2016 Jun 1;8(6):670-8. doi: 10.15252/emmm.201505994. Print 2016 Jun. EMBO Mol Med. 2016. PMID: 27137488 Free PMC article.
-
Oxidative stress, innate immunity, and age-related macular degeneration.AIMS Mol Sci. 2016;3(2):196-221. doi: 10.3934/molsci.2016.2.196. Epub 2016 May 11. AIMS Mol Sci. 2016. PMID: 27239555 Free PMC article.
-
Splenic monocytes drive pathogenic subretinal inflammation in age-related macular degeneration.J Neuroinflammation. 2024 Jan 17;21(1):22. doi: 10.1186/s12974-024-03011-z. J Neuroinflammation. 2024. PMID: 38233865 Free PMC article.
-
Regulation of age-related macular degeneration-like pathology by complement factor H.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):E3040-9. doi: 10.1073/pnas.1424391112. Epub 2015 May 19. Proc Natl Acad Sci U S A. 2015. PMID: 25991857 Free PMC article.
References
-
- Friedman D.S., et al. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122:564–572. - PubMed
-
- Bird A.C., et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 1995;39:367–374. - PubMed
-
- Farkas T.G., Sylvester V., Archer D., Altona M. The histochemistry of drusen. Am. J. Ophthalmol. 1971;71:1206–1215. - PubMed
-
- Hageman G.S., et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20:705–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

