Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion
- PMID: 17909632
- PMCID: PMC1994625
- DOI: 10.1172/JCI32218
Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion
Abstract
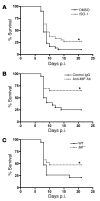
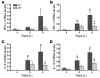
The flavivirus West Nile virus (WNV) is an emerging pathogen that causes life-threatening encephalitis in susceptible individuals. We investigated the role of the proinflammatory cytokine macrophage migration inhibitory factor (MIF), which is an upstream mediator of innate immunity, in WNV immunopathogenesis. We found that patients suffering from acute WNV infection presented with increased MIF levels in plasma and in cerebrospinal fluid. MIF expression also was induced in WNV-infected mice. Remarkably, abrogation of MIF action by 3 distinct approaches (antibody blockade, small molecule pharmacologic inhibition, and genetic deletion) rendered mice more resistant to WNV lethality. Mif(-/-) mice showed a reduced viral load and inflammatory response in the brain when compared with wild-type mice. Our results also indicate that MIF favors viral neuroinvasion by compromising the integrity of the blood-brain barrier. In conclusion, the data obtained from this study provide direct evidence for the involvement of MIF in viral pathogenesis and suggest that pharmacotherapeutic approaches targeting MIF may hold promise for the treatment of WNV encephalitis.
Figures
Similar articles
-
Association between high expression macrophage migration inhibitory factor (MIF) alleles and West Nile virus encephalitis.Cytokine. 2016 Feb;78:51-4. doi: 10.1016/j.cyto.2015.11.021. Epub 2015 Nov 28. Cytokine. 2016. PMID: 26638028 Free PMC article.
-
Cellular microRNA-155 Regulates Virus-Induced Inflammatory Response and Protects against Lethal West Nile Virus Infection.Viruses. 2019 Dec 19;12(1):9. doi: 10.3390/v12010009. Viruses. 2019. PMID: 31861621 Free PMC article.
-
Impaired virus clearance, compromised immune response and increased mortality in type 2 diabetic mice infected with West Nile virus.PLoS One. 2012;7(8):e44682. doi: 10.1371/journal.pone.0044682. Epub 2012 Aug 31. PLoS One. 2012. PMID: 22953001 Free PMC article.
-
Pathogenic roles of macrophage migration inhibitory factor during dengue virus infection.Mediators Inflamm. 2015;2015:547094. doi: 10.1155/2015/547094. Epub 2015 Mar 2. Mediators Inflamm. 2015. PMID: 25821355 Free PMC article. Review.
-
West Nile virus: immunity and pathogenesis.Viruses. 2011 Jun;3(6):811-28. doi: 10.3390/v3060811. Epub 2011 Jun 15. Viruses. 2011. PMID: 21994755 Free PMC article. Review.
Cited by
-
Expression and function of macrophage migration inhibitory factor (MIF) in melioidosis.PLoS Negl Trop Dis. 2010 Feb 16;4(2):e605. doi: 10.1371/journal.pntd.0000605. PLoS Negl Trop Dis. 2010. PMID: 20169062 Free PMC article.
-
Post-translational regulation of macrophage migration inhibitory factor: Basis for functional fine-tuning.Redox Biol. 2018 May;15:135-142. doi: 10.1016/j.redox.2017.11.028. Epub 2017 Dec 6. Redox Biol. 2018. PMID: 29247897 Free PMC article. Review.
-
Southern California neuroinvasive West Nile virus case series.Neurol Sci. 2018 Feb;39(2):251-257. doi: 10.1007/s10072-017-3164-6. Epub 2017 Nov 8. Neurol Sci. 2018. PMID: 29119349
-
Drak2 contributes to West Nile virus entry into the brain and lethal encephalitis.J Immunol. 2008 Aug 1;181(3):2084-91. doi: 10.4049/jimmunol.181.3.2084. J Immunol. 2008. PMID: 18641347 Free PMC article.
-
The structural immunology of antibody protection against West Nile virus.Immunol Rev. 2008 Oct;225:212-25. doi: 10.1111/j.1600-065X.2008.00676.x. Immunol Rev. 2008. PMID: 18837784 Free PMC article. Review.
References
-
- Petersen L.R., Marfin A.A. West Nile virus: a primer for the clinician. Ann. Intern. Med. 2002;137:173–179. - PubMed
-
- Dauphin G., Zientara S., Zeller H., Murgue B. West Nile: worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. Dis. 2004;27:343–355. - PubMed
-
- Granwehr B.P., et al. West Nile virus: where are we now? Lancet Infect. Dis. 2004;4:547–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

