Functional links between Drosophila Nipped-B and cohesin in somatic and meiotic cells
- PMID: 17909832
- PMCID: PMC2258212
- DOI: 10.1007/s00412-007-0125-5
Functional links between Drosophila Nipped-B and cohesin in somatic and meiotic cells
Abstract
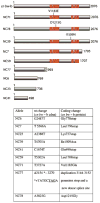
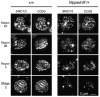
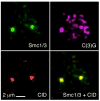
Drosophila Nipped-B is an essential protein that has multiple functions. It facilitates expression of homeobox genes and is also required for sister chromatid cohesion. Nipped-B is conserved from yeast to man, and its orthologs also play roles in deoxyribonucleic acid repair and meiosis. Mutation of the human ortholog, Nipped-B-Like (NIPBL), causes Cornelia de Lange syndrome (CdLS), associated with multiple developmental defects. The Nipped-B protein family is required for the cohesin complex that mediates sister chromatid cohesion to bind to chromosomes. A key question, therefore, is whether the Nipped-B family regulates gene expression, meiosis, and development by controlling cohesin. To gain insights into Nipped-B's functions, we compared the effects of several Nipped-B mutations on gene expression, sister chromatid cohesion, and meiosis. We also examined association of Nipped-B and cohesin with somatic and meiotic chromosomes by immunostaining. Missense Nipped-B alleles affecting the same HEAT repeat motifs as CdLS-causing NIPBL mutations have intermediate effects on both gene expression and mitotic chromatid cohesion, linking these two functions and the role of NIPBL in human development. Nipped-B colocalizes extensively with cohesin on chromosomes in both somatic and meiotic cells and is present in soluble complexes with cohesin subunits in nuclear extracts. In meiosis, Nipped-B also colocalizes with the synaptonemal complex and contributes to maintenance of meiotic chromosome cores. These results support the idea that direct regulation of cohesin function underlies the diverse functions of Nipped-B and its orthologs.
Figures






References
-
- Arumugam P, Gruber S, Tanaka K, Haering CH, Mechtler K, Nasmyth K. ATP hydrolysis is required for cohesin's association with chromosomes. Curr Biol. 2003;13:1941–1953. - PubMed
-
- Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allele of the meiotic cohesin Rec8. Genesis. 2004;40:184–194. - PubMed
-
- Bernard P, Maure JF, Partridge JF, Genier S, Javerzat JP, Allshire RC. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. - PubMed
-
- Bernard P, Drogat J, Maure JF, Dheur S, Vaur S, Genier S, Javerzat JP. A screen for cohesion mutants uncovers Ssl3, the fission yeast counterpart of the cohesin loading factor Scc4. Curr Biol. 2006;16:875–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

