Deregulation of cofactor of BRCA1 expression in breast cancer cells
- PMID: 17910036
- PMCID: PMC8822876
- DOI: 10.1002/jcb.21568
Deregulation of cofactor of BRCA1 expression in breast cancer cells
Abstract
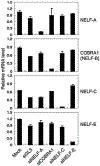
Cofactor of BRCA1 (COBRA1) is an integral component of the human negative elongation factor (NELF), a four-subunit protein complex that inhibits transcription elongation. Previous in vivo work indicates that COBRA1 and the rest of the NELF complex repress estrogen-dependent transcription and the growth of breast cancer cells. In light of the COBRA1 function in breast cancer-related gene expression, we sought to examine regulation of COBRA1 expression in both established breast cancer cell lines and breast carcinoma tissues. We found that COBRA1 expression was inversely correlated with breast cancer progression, as tumor samples of patients who had distant metastasis and local recurrence expressed very low levels of COBRA1 mRNA when compared to those who were disease free for over 10 years (P = 0.0065 and 0.0081, respectively). Using both breast and prostate cancer cell lines, we also explored the possible mechanisms by which COBRA1 expression is regulated. Our results indicate that the protein abundance of COBRA1 and the other NELF subunits are mutually influenced in a tightly coordinated fashion. Small interfering RNA (siRNA) that targeted at one NELF subunit dampened the protein levels of all four subunits. Conversely, ectopic expression of COBRA1 in the knockdown cells partially rescues the co-depletion of the NELF subunits. In addition, our study suggests that a post-transcriptional, proteasome-independent mechanism is involved in the interdependent regulation of the NELF abundance. Furthermore, a lack of COBRA1 expression in breast carcinoma may serve as a useful indicator for poor prognosis.
Figures

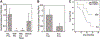
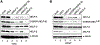
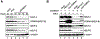
Similar articles
-
Cofactor of BRCA1: a novel transcription factor regulator in upper gastrointestinal adenocarcinomas.Cancer Res. 2006 Feb 1;66(3):1346-53. doi: 10.1158/0008-5472.CAN-05-3593. Cancer Res. 2006. PMID: 16452188
-
Regulation of clustered gene expression by cofactor of BRCA1 (COBRA1) in breast cancer cells.Oncogene. 2007 Apr 19;26(18):2543-53. doi: 10.1038/sj.onc.1210047. Epub 2006 Oct 16. Oncogene. 2007. PMID: 17043641
-
Concerted transcriptional regulation by BRCA1 and COBRA1 in breast cancer cells.Int J Biol Sci. 2007 Nov 26;3(7):486-92. doi: 10.7150/ijbs.3.486. Int J Biol Sci. 2007. PMID: 18071589 Free PMC article.
-
Knockdown of COBRA1 decreases the proliferation and migration of hepatocellular carcinoma cells.Oncol Rep. 2017 Mar;37(3):1896-1906. doi: 10.3892/or.2017.5390. Epub 2017 Jan 19. Oncol Rep. 2017. PMID: 28112367
-
Mouse cofactor of BRCA1 (Cobra1) is required for early embryogenesis.PLoS One. 2009;4(4):e5034. doi: 10.1371/journal.pone.0005034. Epub 2009 Apr 2. PLoS One. 2009. PMID: 19340312 Free PMC article.
Cited by
-
NELF potentiates gene transcription in the Drosophila embryo.PLoS One. 2010 Jul 9;5(7):e11498. doi: 10.1371/journal.pone.0011498. PLoS One. 2010. PMID: 20634899 Free PMC article.
-
Rapid activity-induced transcription of Arc and other IEGs relies on poised RNA polymerase II.Nat Neurosci. 2011 May 29;14(7):848-56. doi: 10.1038/nn.2839. Nat Neurosci. 2011. PMID: 21623364 Free PMC article.
-
Negative role of trihydrophobin 1 in breast cancer growth and migration.Cancer Sci. 2010 Oct;101(10):2156-62. doi: 10.1111/j.1349-7006.2010.01656.x. Epub 2010 Aug 2. Cancer Sci. 2010. PMID: 20735431 Free PMC article.
-
Translational Initiation at a Non-AUG Start Codon for Human and Mouse Negative Elongation Factor-B.PLoS One. 2015 May 26;10(5):e0127422. doi: 10.1371/journal.pone.0127422. eCollection 2015. PLoS One. 2015. PMID: 26010750 Free PMC article.
-
Genetic and genomic analyses of RNA polymerase II-pausing factor in regulation of mammalian transcription and cell growth.J Biol Chem. 2011 Oct 21;286(42):36248-57. doi: 10.1074/jbc.M111.269167. Epub 2011 Aug 24. J Biol Chem. 2011. PMID: 21865163 Free PMC article.
References
-
- Aiyar SE, Blair AL, Hopkinson DA, Bekiranov S, Li R. 2007. Regulation of clustered gene expression by cofactor of BRCA1 (COBRA1) in breast cancer cells. Oncogene 26:2543–2553. - PubMed
-
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, T JM, Meltzer PS. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968. - PubMed
-
- Cai J, Parr C, Jiang WG, Boulton ME. 2006. Expression of pigment epithelial derived factor (PEDF) in human breast cancer and the impact on angiogenesis. Clinical Cancer Res 12:3510–3517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

