Targeted therapy in rectal cancer
- PMID: 17910311
- PMCID: PMC2686129
Targeted therapy in rectal cancer
Abstract
Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) are often overexpressed in colorectal cancer and are associated with inferior outcomes. Based on successful randomized phase III trials, anti-EGFR and anti-VEGF therapeutics have entered clinical practice. Cetuximab (Erbitux), an EGFR-specific antibody, is currently approved in the United States in combination with irinotecan (Camptosar) for patients with metastatic colorectal cancer refractory to irinotecan or as a single agent for patients unable to tolerate irinotecan-based therapy. In retrospective analyses, patients with EGFR-expressing rectal cancer undergoing neoadjuvant radiation therapy had a significantly inferior disease-free survival and lower rates of achieving pathologic complete response. Based on the positive data in metastatic colorectal cancer and synergy with radiation therapy seen in preclinical models, there is a strong rationale to combine cetuximab with neoadjuvant radiation therapy and chemotherapy in rectal cancer. Bevacizumab (Avastin), a VEGF-specific antibody, was the first antiangiogenic agent to be approved in the United States for use in combination with standard chemotherapy in the first- and second-line of treatment in metastatic colorectal cancer. VEGF-targeted therapy may lead to indirect killing of cancer cells by damaging tumor blood vessels, and may increase the radiosensitivity of tumor-associated endothelial cells. VEGF blockade can also "normalize" tumor vasculature, thereby leading to greater tumor oxygenation and drug penetration. This review will address completed and ongoing trials that have established and continue to clarify the effects of these agents in rectal cancer.
Figures

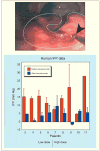
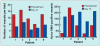
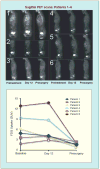

References
-
- Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278–1292. - PubMed
-
- Rich T, Gunderson LL, Lew R, et al. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–1329. - PubMed
-
- Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. - PubMed
-
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. - PubMed
-
- Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

