Side chain and backbone dynamics of phospholamban in phospholipid bilayers utilizing 2H and 15N solid-state NMR spectroscopy
- PMID: 17910421
- PMCID: PMC2756648
- DOI: 10.1021/bi700749q
Side chain and backbone dynamics of phospholamban in phospholipid bilayers utilizing 2H and 15N solid-state NMR spectroscopy
Abstract
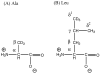
2H and 15N solid-state NMR spectroscopic techniques were used to investigate both the side chain and backbone dynamics of wild-type phospholamban (WT-PLB) and its phosphorylated form (P-PLB) incorporated into 1-palmitoyl-2-oleoyl-sn-glycerophosphocholine (POPC) phospholipid bilayers. 2H NMR spectra of site-specific CD3-labeled WT-PLB (at Leu51, Ala24, and Ala15) in POPC bilayers were similar under frozen conditions (-25 degrees C). However, significant differences in the line shapes of the 2H NMR spectra were observed in the liquid crystalline phase at and above 0 degrees C. The 2H NMR spectra indicate that Leu51, located toward the lower end of the transmembrane (TM) helix, shows restricted side chain motion, implying that it is embedded inside the POPC lipid bilayer. Additionally, the line shape of the 2H NMR spectrum of CD3-Ala24 reveals more side chain dynamics, indicating that this residue (located in the upper end of the TM helix) has additional backbone and internal side chain motions. 2H NMR spectra of both WT-PLB and P-PLB with CD3-Ala15 exhibit strong isotropic spectral line shapes. The dynamic isotropic nature of the 2H peak can be attributed to side chain and backbone motions to residues located in an aqueous environment outside the membrane. Also, the spectra of 15N-labeled amide WT-PLB at Leu51 and Leu42 residues showed only a single powder pattern component indicating that these two 15N-labeled residues located in the TM helix are motionally restricted at 25 degrees C. Conversely, 15N-labeled amide WT-PLB at Ala11 located in the cytoplasmic domain showed both powder and isotropic components at 25 degrees C. Upon phosphorylation, the mobile component contribution increases at Ala11. The 2H and 15N NMR data indicate significant backbone motion for the cytoplasmic domain of WT-PLB when compared to the transmembrane section.
Figures




Similar articles
-
Investigating the dynamic properties of the transmembrane segment of phospholamban incorporated into phospholipid bilayers utilizing 2H and 15N solid-state NMR spectroscopy.Biochemistry. 2004 Nov 9;43(44):13899-909. doi: 10.1021/bi0490993. Biochemistry. 2004. PMID: 15518538
-
The structural topology of wild-type phospholamban in oriented lipid bilayers using 15N solid-state NMR spectroscopy.Protein Sci. 2007 Nov;16(11):2345-9. doi: 10.1110/ps.072977707. Epub 2007 Sep 28. Protein Sci. 2007. PMID: 17905829 Free PMC article.
-
The structural properties of the transmembrane segment of the integral membrane protein phospholamban utilizing (13)C CPMAS, (2)H, and REDOR solid-state NMR spectroscopy.Biochim Biophys Acta. 2006 Jun;1758(6):772-80. doi: 10.1016/j.bbamem.2006.04.016. Epub 2006 May 19. Biochim Biophys Acta. 2006. PMID: 16839519
-
Direct spectroscopic detection of molecular dynamics and interactions of the calcium pump and phospholamban.Ann N Y Acad Sci. 1998 Sep 16;853:186-94. doi: 10.1111/j.1749-6632.1998.tb08266.x. Ann N Y Acad Sci. 1998. PMID: 10603946 Review.
-
Structure determination of membrane proteins in five easy pieces.Methods. 2011 Dec;55(4):363-9. doi: 10.1016/j.ymeth.2011.09.009. Epub 2011 Sep 20. Methods. 2011. PMID: 21964394 Free PMC article. Review.
Cited by
-
Deuterium magic angle spinning NMR used to study the dynamics of peptides adsorbed onto polystyrene and functionalized polystyrene surfaces.J Phys Chem B. 2011 Aug 4;115(30):9452-60. doi: 10.1021/jp1101829. Epub 2011 Jul 11. J Phys Chem B. 2011. PMID: 21650191 Free PMC article.
-
Probing ground and excited states of phospholamban in model and native lipid membranes by magic angle spinning NMR spectroscopy.Biochim Biophys Acta. 2012 Feb;1818(2):146-53. doi: 10.1016/j.bbamem.2011.07.040. Epub 2011 Aug 3. Biochim Biophys Acta. 2012. PMID: 21839724 Free PMC article.
-
Partitioning, dynamics, and orientation of lung surfactant peptide KL(4) in phospholipid bilayers.Biochim Biophys Acta. 2010 Feb;1798(2):216-22. doi: 10.1016/j.bbamem.2009.08.020. Epub 2009 Sep 6. Biochim Biophys Acta. 2010. PMID: 19735643 Free PMC article.
-
(15)N Solid-state NMR spectroscopic studies on phospholamban at its phosphorylated form at ser-16 in aligned phospholipid bilayers.Biochim Biophys Acta. 2010 Mar;1798(3):312-7. doi: 10.1016/j.bbamem.2009.12.020. Epub 2010 Jan 4. Biochim Biophys Acta. 2010. PMID: 20044975 Free PMC article.
-
Probing the helical tilt and dynamic properties of membrane-bound phospholamban in magnetically aligned bicelles using electron paramagnetic resonance spectroscopy.Biochim Biophys Acta. 2012 Mar;1818(3):645-50. doi: 10.1016/j.bbamem.2011.11.030. Epub 2011 Dec 4. Biochim Biophys Acta. 2012. PMID: 22172806 Free PMC article.
References
-
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban: Identification of phosphorylation sites and two major structural domains. J. Biol. Chem. 1986;261:13333–13341. - PubMed
-
- Simmerman HKB, Kobayashi YM, Autry JM, Jones LR. A leucine zipper stabilizes the pentameric membrane domain of phospholamban and forms a coiled-coil pore structure. J. Biol. Chem. 1996;271:5941–5946. - PubMed
-
- Simmerman HKB, Jones LR. Phospholamban: Protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 1998;78:921–947. - PubMed
-
- James P, Inui M, Tada M, Chiesi M, Carafoli E. Nature and site of phospholamban regulation of the calcium pump of sarcoplasmic reticulum. Nature. 1989;342:90–92. - PubMed
-
- Kirchberger MA, Tada M, Katz AM. Phospholamban a regulatory protein of the cardiac sarcoplasmic reticulum. Recent Adv. Stud. Card. Struct. Metab. 1975;5:103–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

