Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1
- PMID: 17911102
- PMCID: PMC3909483
- DOI: 10.1074/jbc.M705823200
Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1
Abstract
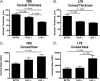
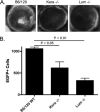
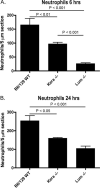
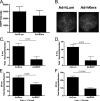
Keratocan and lumican are keratan-sulfate proteoglycans (KSPG), which have a critical role in maintaining corneal clarity. To determine whether these KSPGs have a role in corneal inflammation, we examined Kera(-/-) and Lum(-/-) mice in a model of lipopolysaccharide (LPS)-induced keratitis in which wild-type mice develop increased corneal thickness and haze due to neutrophil infiltration to the corneal stroma. Corneal thickness increases caused by LPS mice were significantly lower in Kera(-/-) and Lum(-/-) than wild-type mice. Further, LPS-injected Lum(-/-) mice had elevated corneal haze levels compared with that of Kera(-/-) and wild-type. At 24 h post-injection, total enhanced green fluorescent protein-positive bone marrow-derived inflammatory cells in chimeric mice was significantly lower in Kera(-/-) mice and Lum(-/-) mice compared with wild-type mice. Neutrophil infiltration was inhibited in Kera(-/-) and Lum(-/-) mice at 6 and 24 h post-stimulation, with Lum(-/-) corneas having the most profound defect in neutrophil migration. Reconstitution of keratocan and lumican expression in corneas of Kera(-/-) and Lum(-/-) mice using adeno-keratocan and adeno-lumican viral vectors, respectively, resulted in normal neutrophil infiltration in response to LPS. Immunoprecipitation/Western blot analysis showed that lumican and keratocan core proteins bind the CXC chemokine KC during a corneal inflammatory response, indicating that corneal KSPGs mediate neutrophil recruitment to the cornea by regulating chemokine gradient formation. Together, these data support a significant role for lumican and keratocan in a corneal inflammatory response with respect to edema, corneal clarity, and cellular infiltration.
Figures


Similar articles
-
Regulation of corneal inflammation by neutrophil-dependent cleavage of keratan sulfate proteoglycans as a model for breakdown of the chemokine gradient.J Leukoc Biol. 2010 Sep;88(3):517-22. doi: 10.1189/jlb.0310134. Epub 2010 May 21. J Leukoc Biol. 2010. PMID: 20495072 Free PMC article.
-
Targeted expression of a lumican transgene rescues corneal deficiencies in lumican-null mice.Mol Vis. 2007 Oct 18;13:2012-8. Mol Vis. 2007. PMID: 17982425
-
Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican.J Biol Chem. 2005 Jul 8;280(27):25541-7. doi: 10.1074/jbc.M500249200. Epub 2005 Apr 22. J Biol Chem. 2005. PMID: 15849191 Free PMC article.
-
Roles of lumican and keratocan on corneal transparency.Glycoconj J. 2002 May-Jun;19(4-5):275-85. doi: 10.1023/A:1025396316169. Glycoconj J. 2002. PMID: 12975606 Review.
-
Functions of lumican and fibromodulin: lessons from knockout mice.Glycoconj J. 2002 May-Jun;19(4-5):287-93. doi: 10.1023/A:1025348417078. Glycoconj J. 2002. PMID: 12975607 Review.
Cited by
-
Bone marrow chimeras and c-fms conditional ablation (Mafia) mice reveal an essential role for resident myeloid cells in lipopolysaccharide/TLR4-induced corneal inflammation.J Immunol. 2009 Mar 1;182(5):2738-44. doi: 10.4049/jimmunol.0803505. J Immunol. 2009. PMID: 19234168 Free PMC article.
-
Rational design and synthesis of lumican stapled peptides for promoting corneal wound healing.Ocul Surf. 2023 Oct;30:168-178. doi: 10.1016/j.jtos.2023.09.007. Epub 2023 Sep 22. Ocul Surf. 2023. PMID: 37742739 Free PMC article.
-
Regulation of corneal inflammation by neutrophil-dependent cleavage of keratan sulfate proteoglycans as a model for breakdown of the chemokine gradient.J Leukoc Biol. 2010 Sep;88(3):517-22. doi: 10.1189/jlb.0310134. Epub 2010 May 21. J Leukoc Biol. 2010. PMID: 20495072 Free PMC article.
-
Exploration of Mediators Associated with Myocardial Remodelling in Feline Hypertrophic Cardiomyopathy.Animals (Basel). 2023 Jun 26;13(13):2112. doi: 10.3390/ani13132112. Animals (Basel). 2023. PMID: 37443910 Free PMC article.
-
Stem cell therapy restores transparency to defective murine corneas.Stem Cells. 2009 Jul;27(7):1635-42. doi: 10.1002/stem.91. Stem Cells. 2009. PMID: 19544455 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

