Insights into how to conduct a clinical trial in the UK
- PMID: 17911130
- PMCID: PMC1997262
- DOI: 10.1177/014107680710001016
Insights into how to conduct a clinical trial in the UK
Abstract
New researchers may find starting and conducting clinical studies in the UK complicated and time-consuming. In this article, we describe our collective experiences and provide some pointers on how to navigate through the various committees and regulatory bodies. The article is intended to aid junior researchers in understanding the study process and to provide them with some insight on how to get through this complex system successfully.
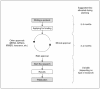
Figures
Similar articles
-
American Society of Clinical Oncology policy statement: oversight of clinical research.J Clin Oncol. 2003 Jun 15;21(12):2377-86. doi: 10.1200/JCO.2003.04.026. Epub 2003 Apr 29. J Clin Oncol. 2003. PMID: 12721281
-
Ethics of private panels comes under scrutiny.Nature. 1997 May 29;387(6632):445. doi: 10.1038/387445a0. Nature. 1997. PMID: 9168099 No abstract available.
-
The reform of UK research ethics committees: throwing the baby out with the bath water?J Med Ethics. 2005 Aug;31(8):487-9. doi: 10.1136/jme.2004.010546. J Med Ethics. 2005. PMID: 16076976 Free PMC article.
-
Gaining approval for clinical research.Br J Hosp Med (Lond). 2016 Jul;77(7):414-8. doi: 10.12968/hmed.2016.77.7.414. Br J Hosp Med (Lond). 2016. PMID: 27388381 Review.
-
Ethics approval, guarantees of quality and the meddlesome editor.J Clin Nurs. 2007 Aug;16(8):1398-404. doi: 10.1111/j.1365-2702.2006.01918.x. J Clin Nurs. 2007. PMID: 17655528 Review.
Cited by
-
Applying for regulatory approval of a clinical trial of a medical device in the UK - A practical guide.Br Dent J. 2018 Dec 14;225(11):1033-1036. doi: 10.1038/sj.bdj.2018.1033. Br Dent J. 2018. PMID: 30547908
-
Streamlining and cycle time reduction of the startup phase of clinical trials.Trials. 2020 Jan 29;21(1):115. doi: 10.1186/s13063-020-4079-8. Trials. 2020. PMID: 31996249 Free PMC article.
References
-
- Department of Health. Research and Development for a First Class Service. London: DoH, 2000. Available at http://www.dh.gov.uk
-
- Anderson JJ, Felson DT, Meenan RF. Secular changes in published clinical trials of second-line agents in rheumatoid arthritis. Arthritis Rheum 1991;34: 1304-9 - PubMed
-
- Dorman PJ, Counsell C, Sandercock P. Reports of randomized trials in acute stroke, 1955 to 1995. What proportions were commercially sponsored? Stroke 1999;30: 1995-8 - PubMed
-
- Department of Health. Report of the Ad Hoc Advisory Group on the Operation of NHS Research Ethics Committees. London: DoH, 2005. Available at http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/Publicati...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical