Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development
- PMID: 17911264
- PMCID: PMC2042180
- DOI: 10.1073/pnas.0703942104
Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development
Abstract
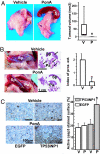
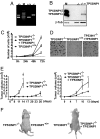
Pancreatic cancer is a disease with an extremely poor prognosis. Tumor protein 53-induced nuclear protein 1 (TP53INP1) is a proapoptotic stress-induced p53 target gene. In this article, we show by immunohistochemical analysis that TP53INP1 expression is dramatically reduced in pancreatic ductal adenocarcinoma (PDAC) and this decrease occurs early during pancreatic cancer development. TP53INP1 reexpression in the pancreatic cancer-derived cell line MiaPaCa2 strongly reduced its capacity to form s.c., i.p., and intrapancreatic tumors in nude mice. This anti-tumoral capacity is, at least in part, due to the induction of caspase 3-mediated apoptosis. In addition, TP53INP1(-/-) mouse embryonic fibroblasts (MEFs) transformed with a retrovirus expressing E1A/ras(V12) oncoproteins developed bigger tumors than TP53INP1(+/+) transformed MEFs or TP53INP1(-/-) transformed MEFs with restored TP53INP1 expression. Finally, TP53INP1 expression is repressed by the oncogenic micro RNA miR-155, which is overexpressed in PDAC cells. TP53INP1 is a previously unknown miR-155 target presenting anti-tumoral activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, DePinho RA. Genes Dev. 2006;20:1218–1249. - PubMed
-
- Tomasini R, Samir AA, Vaccaro MI, Pebusque MJ, Dagorn JC, Iovanna JL, Dusetti NJ. J Biol Chem. 2001;276:44185–44192. - PubMed
-
- Tomasini R, Samir AA, Pebusque MJ, Calvo EL, Totaro S, Dagorn JC, Dusetti NJ, Iovanna JL. Eur J Cell Biol. 2002;81:294–301. - PubMed
-
- Tomasini R, Samir AA, Carrier A, Isnardon D, Cecchinelli B, Soddu S, Malissen B, Dagorn JC, Iovanna JL, Dusetti NJ. J Biol Chem. 2003;278:37722–37729. - PubMed
-
- Yoshida K, Liu H, Miki Y. J Biol Chem. 2006;281:5734–5740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

