Sex-dependent up-regulation of two splicing factors, Psf and Srp20, during hippocampal memory formation
- PMID: 17911373
- PMCID: PMC2044560
- DOI: 10.1101/lm.640307
Sex-dependent up-regulation of two splicing factors, Psf and Srp20, during hippocampal memory formation
Abstract
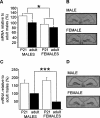
Gene transcription is required for long-term memory (LTM) formation. LTM formation is impaired in a male-specific manner in mice lacking either of the two Ca(2+)/calmodulin-dependent kinase kinase (Camkk) genes. Since altered transcription was suggested to cause these impairments in LTM formation, we used microarrays to screen for CaMKKbeta-dependent gene expression changes. Here we show that the hippocampal mRNA expression of two splicing factors, splicing factor arginine/serine-rich 3 (Sfrs3/Srp20) and polypyrimidine tract-binding protein-associated splicing factor (Psf), is altered in CaMKKbeta-deficient males. In wild-type (WT) mice, the basal expression level in the hippocampus is higher in males than in females, and the sex difference in Srp20 expression is detectable before puberty. Training in two hippocampus-dependent learning tasks, the spatial version of the Morris water maze (MWM) and background contextual fear conditioning, increases the hippocampal mRNA expression of both splicing factors in WT males. However, the increase in Srp20 mRNA expression occurs only in males and not in females, whereas the up-regulation of Psf expression occurs in both sexes. Importantly, control experiments demonstrate that the up-regulation of both splicing factors is specific for the learned associations after contextual fear conditioning. In summary, we provide the first evidence for a regulation of splicing factors during LTM formation and we suggest that alternative splicing contributes to sex differences in LTM formation.
Figures





References
-
- Aguilar-Valles A., Sanchez E., deGortari P., Balderas I., Ramirez-Amaya V., Bermudez-Rattoni F., Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: Differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82:306–319. - PubMed
-
- Angelo M., Plattner F., Irvine E.E., Giese K.P. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur. J. Neurosci. 2003;18:423–431. - PubMed
-
- Beffert U., Weeber E.J., Durudas A., Qiu S., Masiulis I., Sweatt J.D., Li W.P., Adelmann G., Frotscher M., Hammer R.E., et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. - PubMed
-
- Bladen C.L., Udayakumar D., Takeda Y., Dynan W.S. Identification of the polypyrimidine tract binding protein-associated splicing factor.p54(nrb) complex as a candidate DNA double-strand break rejoining factor. J. Biol. Chem. 2005;280:5205–5210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
