PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection
- PMID: 17911605
- PMCID: PMC2291549
- DOI: 10.4049/jimmunol.179.8.5204
PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection
Abstract
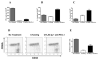
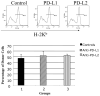
The PD-1:PDL pathway plays an important role in regulating alloimmune responses but its role in transplantation tolerance is unknown. We investigated the role of PD-1:PDL costimulatory pathway in peripheral and a well established model of central transplantation tolerance. Early as well as delayed blockade of PDL1 but not PDL2 abrogated tolerance induced by CTLA4Ig in a fully MHC-mismatched cardiac allograft model. Accelerated rejection was associated with a significant increase in the frequency of IFN-gamma-producing alloreactive T cells and expansion of effector CD8(+) T cells in the periphery, and a decline in the percentage of Foxp3(+) graft infiltrating cells. Similarly, studies using PDL1/L2-deficient recipients confirmed the results with Ab blockade. Interestingly, while PDL1-deficient donor allografts were accepted by wild-type recipients treated with CTLA4Ig, the grafts developed severe chronic rejection and vasculopathy when compared with wild-type grafts. Finally, in a model of central tolerance induced by mixed allogeneic chimerism, engraftment was not abrogated by PDL1/L2 blockade. These novel data demonstrate the critical role of PDL1 for induction and maintenance of peripheral transplantation tolerance by its ability to alter the balance between pathogenic and regulatory T cells. Expression of PDL1 in donor tissue is critical for prevention of in situ graft pathology and chronic rejection.
Figures





References
-
- Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol. Rev. 2003;196:85–108. - PubMed
-
- Pearson TC, Alexander DZ, Hendrix R, Elwood ET, Linsley PS, Winn KJ, Larsen CP. CTLA4Ig plus bone marrow induces longterm allograft survival and donor specific unresponsiveness in the murine model. Evidence for hematopoietic chimerism. Transplantation. 1996;61:997–1004. - PubMed
-
- Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4Ig. Transplantation. 1994;57:1701–1706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI056299/AI/NIAID NIH HHS/United States
- 2 R37 HL56067/HL/NHLBI NIH HHS/United States
- R01 AI051559/AI/NIAID NIH HHS/United States
- P01 AI041521/AI/NIAID NIH HHS/United States
- R01-HL63452/HL/NHLBI NIH HHS/United States
- P01-AI041521/AI/NIAID NIH HHS/United States
- R37 HL056067/HL/NHLBI NIH HHS/United States
- K08 AI064335/AI/NIAID NIH HHS/United States
- P01-AI56299/AI/NIAID NIH HHS/United States
- 1K08AI064335/AI/NIAID NIH HHS/United States
- R01-AI34495/AI/NIAID NIH HHS/United States
- R01-AI51559/AI/NIAID NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- R01 HL063452/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

