Mucin impedes cytotoxic effect of 5-FU against growth of human pancreatic cancer cells: overcoming cellular barriers for therapeutic gain
- PMID: 17912239
- PMCID: PMC2360416
- DOI: 10.1038/sj.bjc.6603972
Mucin impedes cytotoxic effect of 5-FU against growth of human pancreatic cancer cells: overcoming cellular barriers for therapeutic gain
Abstract
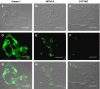
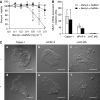
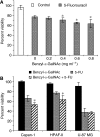
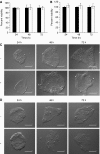
Mucins are high molecular weight glycoproteins expressed on the apical surface of normal epithelial cells. In cancer disease mucins are overexpressed on the entire cellular surface. Overexpression of MUC1 mucin in pancreatic tumours has been correlated with poor patient survival. Current chemotherapeutic approaches such as 5-fluorouracil (5-FU) has produced limited clinical success. In this study we investigated the role of mucin in cytotoxic drug treatment to determine whether the extracellular domain of mucin impedes cytotoxic drug action of 5-FU. Human pancreatic cancer cells revealed high and relatively moderate MUC1 levels for Capan-1 and HPAF-II, respectively, compared to MUC1 negative control (U-87 MG glioblastoma) that showed relatively non-specific anti-MUC1 uptake. Benzyl-alpha-GalNAc (O-glycosylation inhibitor) was used to reduce mucin on cell surfaces, and neuraminidase was used to hydrolyse sialic acid at the distal end of carbohydrate chains. Benzyl-alpha-GalNAc had no effect on cell morphology or proliferation at the concentrations employed. The inhibition of O-glycosylation resulted in significant 5-FU antiproliferative activity against Capan-1 and HPAF-II, but not against U-87 MG. However, the exposure of cells to neuraminidase failed to improve the cytotoxic action of 5-FU. Our experimental findings suggest that the overexpression of mucin produced by human pancreatic tumours might limit the effectiveness of chemotherapy.
Figures

Similar articles
-
Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours.Eur J Cancer. 2009 Jan;45(1):164-73. doi: 10.1016/j.ejca.2008.10.008. Epub 2008 Dec 4. Eur J Cancer. 2009. PMID: 19046630
-
Inhibition of KL-6/MUC1 glycosylation limits aggressive progression of pancreatic cancer.World J Gastroenterol. 2014 Sep 14;20(34):12171-81. doi: 10.3748/wjg.v20.i34.12171. World J Gastroenterol. 2014. PMID: 25232251 Free PMC article.
-
Inhibition of the O-glycan elongation limits MUC1 incorporation to cell membrane of human endometrial carcinoma cells.Int J Mol Med. 2004 Mar;13(3):459-64. Int J Mol Med. 2004. PMID: 14767580
-
Diverse glycosylation of MUC1 and MUC2: potential significance in tumor immunity.J Biochem. 1999 Dec;126(6):975-85. doi: 10.1093/oxfordjournals.jbchem.a022565. J Biochem. 1999. PMID: 10578046 Review.
-
Expression of mucin antigens in human cancers and its relationship with malignancy potential.Pathol Int. 1997 Dec;47(12):813-30. doi: 10.1111/j.1440-1827.1997.tb03713.x. Pathol Int. 1997. PMID: 9503463 Review.
Cited by
-
Exploring New Frontiers: Alternative Breast Cancer Treatments Through Glycocalyx Research.Breast J. 2025 May 22;2025:9952727. doi: 10.1155/tbj/9952727. eCollection 2025. Breast J. 2025. PMID: 40443562 Free PMC article. Review.
-
Tumour growth and resistance to gemcitabine of pancreatic cancer cells are decreased by AP-2alpha overexpression.Br J Cancer. 2009 Aug 18;101(4):637-44. doi: 10.1038/sj.bjc.6605190. Br J Cancer. 2009. PMID: 19672266 Free PMC article.
-
Association between aberrant dynein cytoplasmic 1 light intermediate chain 1 expression levels, mucins and chemosensitivity in colorectal cancer.Mol Med Rep. 2020 Jul;22(1):185-192. doi: 10.3892/mmr.2020.11086. Epub 2020 Apr 22. Mol Med Rep. 2020. PMID: 32319648 Free PMC article.
-
Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy.Am J Cancer Res. 2017 Jun 1;7(6):1372-1383. eCollection 2017. Am J Cancer Res. 2017. PMID: 28670497 Free PMC article.
-
PP2A Promotes the Symmetric Division of MUC1-Dominant Cancer Stem-Like Cells in Small Cell Lung Cancer.Adv Sci (Weinh). 2025 Jul;12(25):e2503545. doi: 10.1002/advs.202503545. Epub 2025 May 11. Adv Sci (Weinh). 2025. PMID: 40349179 Free PMC article.
References
-
- American Cancer Society, C.F.F (2007). American Cancer Society: Atlanta. 16–17
-
- Beum PV, Singh J, Burdick M, Hollingsworth MA, Cheng PW (1999) Expression of core 2 beta-1,6-N-acetylglucosaminyltransferase in a human pancreatic cancer cell line results in altered expression of MUC1 tumor-associated epitopes. J Biol Chem 274: 24641–24648 - PubMed
-
- Byrd JC, Dahiya R, Huang J, Kim YS (1995) Inhibition of mucin synthesis by benzyl-alpha-GalNAc in KATO III gastric cancer and Caco-2 colon cancer cells. Eur J Cancer 31A: 1498–1505 - PubMed
-
- Corfield AP, Carroll D, Myerscough N, Probert CS (2001) Mucins in the gastrointestinal tract in health and disease. Front Biosci 6: D1321–D1357 - PubMed
-
- de Cremoux P, Extra JM, Denis MG, Pierga JY, Bourstyn E, Nos C, Clough KB, Boudou E, Martin EC, Muller A, Pouillart P, Magdelenat H (2000) Detection of MUC1-expressing mammary carcinoma cells in the peripheral blood of breast cancer patients by real-time polymerase chain reaction. Clin Cancer Res 6: 3117–3122 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

