Pemetrexed pharmacokinetics and pharmacodynamics in a phase I/II study of doublet chemotherapy with vinorelbine: implications for further optimisation of pemetrexed schedules
- PMID: 17912246
- PMCID: PMC2360430
- DOI: 10.1038/sj.bjc.6603995
Pemetrexed pharmacokinetics and pharmacodynamics in a phase I/II study of doublet chemotherapy with vinorelbine: implications for further optimisation of pemetrexed schedules
Abstract
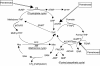
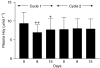
The purpose of this study was to investigate the utility of plasma pharmacokinetic and pharmacodynamic measures including plasma deoxynucleosides, homocysteine and methylmalonic acid concentrations in understanding the time course and extent of the inhibition of thymidylate synthase (TS) by pemetrexed in the context of a phase I/II combination study with vinorelbine. Eighteen patients received supplementation with folic acid and Vitamin B(12) 1 week before beginning treatment with pemetrexed and vinorelbine administered in a dose-escalating manner on a 21-day cycle. Heparinised blood samples were collected from consenting patients in the first cycle for pharmacokinetic analyses and in the first two cycles for determination of plasma thymidine, deoxyuridine, homocysteine and methylmalonic acid concentrations. These values were correlated with response and toxicity. Plasma deoxyuridine was used as a measure of TS inhibition, and concentrations of deoxyuridine were significantly elevated relative to baseline on days 1 (P<0.01), 2 (P<0.001) and 3 (P<0.05) after treatment at all pemetrexed dose levels (400-700 mg m(-2)). The magnitude of deoxyuridine elevation correlated with pemetrexed area under the plasma concentration-time curve (AUC) (r(2)=0.23, P<0.05). However, deoxyuridine concentrations returned to baseline between 8 and 15 days after treatment with pemetrexed, suggesting that inhibition of TS was not durable. Pemetrexed AUC correlated with the percentage decline (relative to baseline) in both platelets (r(2)=0.58, P<0.001) and leucocytes (r(2)=0.26, P<0.05) at day 8. Baseline homocysteine was also significantly correlated with these measures of haematological toxicity (r(2)=0.37, P<0.01 and r(2)=0.39, P<0.01, respectively). In addition, there was a significant reduction of plasma homocysteine on days 8 (P<0.005) and 15 (P<0.05) in cycle 1 compared to baseline values. The results suggest that the TS inhibitory effects of pemetrexed are short-lived and make the case for a more frequent schedule of administration such as every 2 weeks. The lack of protracted TS inhibition may be due to concomitant vitamin administration, and this may be the mechanism by which vitamins prevent life-threatening toxicity from pemetrexed. Baseline homocysteine concentration remains a predictive marker for haematological toxicity even following folate supplementation.
Figures
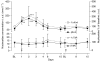

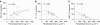
Similar articles
-
A phase I/II study of pemetrexed and vinorelbine in patients with non-small cell lung cancer.Lung Cancer. 2005 Sep;49(3):401-12. doi: 10.1016/j.lungcan.2005.04.003. Lung Cancer. 2005. PMID: 15923057 Clinical Trial.
-
A semimechanistic-physiologic population pharmacokinetic/pharmacodynamic model for neutropenia following pemetrexed therapy.Cancer Chemother Pharmacol. 2006 Apr;57(4):412-26. doi: 10.1007/s00280-005-0077-5. Epub 2005 Dec 2. Cancer Chemother Pharmacol. 2006. PMID: 16322990
-
Phase I and pharmacokinetic study of pemetrexed plus cisplatin in chemonaive patients with locally advanced or metastatic malignant pleural mesothelioma or non-small cell lung cancer.Clin Cancer Res. 2009 Jan 1;15(1):382-9. doi: 10.1158/1078-0432.CCR-08-0128. Clin Cancer Res. 2009. PMID: 19118069 Clinical Trial.
-
Pemetrexed safety and dosing strategy.Semin Oncol. 2002 Dec;29(6 Suppl 18):24-9. doi: 10.1053/sonc.2002.37465. Semin Oncol. 2002. PMID: 12571807 Review.
-
Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent.Clin Cancer Res. 2004 Jun 15;10(12 Pt 2):4276s-4280s. doi: 10.1158/1078-0432.CCR-040010. Clin Cancer Res. 2004. PMID: 15217974 Review.
Cited by
-
Arachidonic acid drives adaptive responses to chemotherapy-induced stress in malignant mesothelioma.J Exp Clin Cancer Res. 2021 Nov 2;40(1):344. doi: 10.1186/s13046-021-02118-y. J Exp Clin Cancer Res. 2021. PMID: 34727953 Free PMC article.
-
Adaptation of a chemosensitivity assay to accurately assess pemetrexed in ex vivo cultures of lung cancer.Cancer Biol Ther. 2013 Jan;14(1):39-44. doi: 10.4161/cbt.22622. Epub 2012 Oct 31. Cancer Biol Ther. 2013. PMID: 23114649 Free PMC article.
-
Phosphodiesterase Inhibition to Sensitize Non-Small-Cell Lung Cancer to Pemetrexed: A Double-Edged Strategy.Cancers (Basel). 2024 Jul 6;16(13):2475. doi: 10.3390/cancers16132475. Cancers (Basel). 2024. PMID: 39001537 Free PMC article.
-
A Supramolecular Nanoparticle of Pemetrexed Improves the Anti-Tumor Effect by Inhibiting Mitochondrial Energy Metabolism.Front Bioeng Biotechnol. 2021 Dec 21;9:804747. doi: 10.3389/fbioe.2021.804747. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34993192 Free PMC article.
-
Correlation of toxicities and efficacies of pemetrexed with clinical factors and single-nucleotide polymorphisms: a prospective observational study.BMC Cancer. 2023 Aug 26;23(1):800. doi: 10.1186/s12885-023-11257-8. BMC Cancer. 2023. PMID: 37633908 Free PMC article.
References
-
- Adjei AA (2003) Pemetrexed (Alimta): a novel multitargeted antifolate agent. Expert Rev Anticancer Ther 3: 145–155 - PubMed
-
- Billion S, Tribout B, Cadet E, Queinnec C, Rochette J, Wheatley P, Bataille P (2002) Hyperhomocysteinaemia, folate and vitamin B12 in unsupplemented haemodialysis patients: effect of oral therapy with folic acid and vitamin B12. Nephrol Dial Transplant 17: 455–461 - PubMed
-
- Bronstrup A, Hages M, Prinz-Langenohl R, Pietrzik K (1998) Effects of folic acid and combinations of folic acid and vitamin B-12 on plasma homocysteine concentrations in healthy, young women. Am J Clin Nutr 68: 1104–1110 - PubMed
-
- Bunn P, Paoletti P, Niyikiza C, Rusthoven J, Nelson K, Hanauske AR, Stabler S, Calvert AH, Allen R (2001) Vitamin B12 and folate reduce toxicity of Alimta™ (Pemetrexed Disodium, LY231514, MTA), a novel antifolate/antimetabolite. Proc Am Soc Clin Oncol 20: 300
-
- Clarke SJ, Boyer MJ, Millward M, Underhill C, Moylan E, Yip D, White S, Childs A, Beale P, Latz J (2005) A phase I/II study of pemetrexed and vinorelbine in patients with non-small cell lung cancer. Lung Cancer 49: 401–412 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

