Role of FGFR3 in urothelial cell carcinoma: biomarker and potential therapeutic target
- PMID: 17912529
- PMCID: PMC4876910
- DOI: 10.1007/s00345-007-0213-4
Role of FGFR3 in urothelial cell carcinoma: biomarker and potential therapeutic target
Abstract
Although non-invasive bladder tumours (pTa) are the most common group of bladder tumours at presentation, there has until recently been relatively little information on their molecular biology. Thus it was of great interest when mutations in the FGF receptor 3 (FGFR3) were identified in bladder tumours and it became apparent that these were most common in tumours of low grade and stage. Since the initial description of activating mutations of FGFR3, there have been numerous studies confirming the frequency and spectrum of these mutations in bladder cancers of all grades and stages. Mutation screening techniques have evolved and improved. FGFR3 mutation has been assessed as a predictive biomarker in tumour tissues and as a diagnostic biomarker in urine. Efforts have been made to understand the function of FGFR3 in urothelial and other cells. Although our understanding of FGFR3 function is incomplete, it is already apparent that this may represent an important therapeutic target not only in non-invasive bladder cancer but also in a significant number of invasive tumours. This review summarises the current state of knowledge of this interesting receptor in urothelial carcinoma (UC).
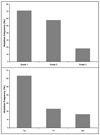
Figures




References
-
- Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. - PubMed
-
- Avivi A, Yayon A, Givol D. A novel form of FGF receptor-3 using an alternative exon in the immunoglobulin domain III. FEBS Lett. 1993;330:249–52. - PubMed
-
- Ornitz DM, Xu J, Colvin JS, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–7. - PubMed
-
- Chellaiah AT, McEwen DG, Werner S, Xu J, Ornitz DM. Fibroblast growth factor receptor (FGFR) 3. Alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J Biol Chem. 1994;269:11620–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

