Letrozole as upfront endocrine therapy for postmenopausal women with hormone-sensitive breast cancer: BIG 1-98
- PMID: 17912636
- PMCID: PMC2001218
- DOI: 10.1007/s10549-007-9700-y
Letrozole as upfront endocrine therapy for postmenopausal women with hormone-sensitive breast cancer: BIG 1-98
Erratum in
- Breast Cancer Res Treat. 2008 Nov;112(2):387
Abstract
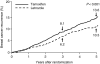
The BIG 1-98 trial is a large, randomized, independently conducted clinical trial designed to compare the efficacy of upfront letrozole versus tamoxifen monotherapy and to compare sequential or up-front use of letrozole and/or tamoxifen as an early adjuvant therapy for patients with early breast cancer. We report on the results from the primary core analysis of the BIG 1-98 trial of 8,010 patients, which compares monotherapy with letrozole versus tamoxifen. This pre-planned core analysis allowed the use of patient data from the monotherapy arms of letrozole and tamoxifen and from the sequential arms prior to the drug switch point. Patients randomized to letrozole had a 19% improved disease-free survival (hazard ratio [HR]=0.81; P=0.003), due especially to reduced distant metastases (HR=0.73; P=0.001). A 14% risk reduction of fatal events in favor of letrozole was also observed (P=NS). The results from the monotherapy arms alone confirmed the findings from the primary core analysis. Based on the results from this trial, the aromatase inhibitor letrozole (Femara) is currently recommended as a part of standard adjuvant therapy for postmenopausal women with endocrine-responsive breast cancer and has recently been approved in the early adjuvant setting in both Europe and the United States. A subsequent analysis after additional follow-up will address the question of monotherapy versus sequential therapy.
Figures



Similar articles
-
Update of the BIG 1-98 Trial: where do we stand?Breast. 2009 Oct;18 Suppl 3:S78-82. doi: 10.1016/S0960-9776(09)70278-4. Breast. 2009. PMID: 19914548 Clinical Trial.
-
A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer.N Engl J Med. 2005 Dec 29;353(26):2747-57. doi: 10.1056/NEJMoa052258. N Engl J Med. 2005. PMID: 16382061 Clinical Trial.
-
Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98.J Clin Oncol. 2007 Feb 10;25(5):486-92. doi: 10.1200/JCO.2006.08.8617. Epub 2007 Jan 2. J Clin Oncol. 2007. PMID: 17200148 Clinical Trial.
-
Understanding the BIG results: Insights from the BIG 1-98 trial analyses.Adv Ther. 2008 Dec;25(12):1257-75. doi: 10.1007/s12325-008-0128-5. Adv Ther. 2008. PMID: 19096768 Review.
-
Letrozole as adjuvant endocrine therapy in postmenopausal women with breast cancer.Expert Rev Anticancer Ther. 2006 Jan;6(1):5-10. doi: 10.1586/14737140.6.1.5. Expert Rev Anticancer Ther. 2006. PMID: 16375638 Review.
Cited by
-
Letrozole: a review of its use in the treatment of postmenopausal women with hormone-responsive early breast cancer.Drugs. 2009 Aug 20;69(12):1681-705. doi: 10.2165/10482340-000000000-00000. Drugs. 2009. PMID: 19678717 Review.
-
Hot flashes: a review of pathophysiology and treatment modalities.Oncologist. 2011;16(11):1658-64. doi: 10.1634/theoncologist.2011-0174. Epub 2011 Oct 31. Oncologist. 2011. PMID: 22042786 Free PMC article. Review.
-
Interpreting Breast International Group (BIG) 1-98: a randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive, early breast cancer.Breast Cancer Res. 2011 May 26;13(3):209. doi: 10.1186/bcr2837. Breast Cancer Res. 2011. PMID: 21635709 Free PMC article. Review.
-
Integrating alternative therapies in overcoming chemotherapy resistance in tumors.Mol Biol Rep. 2025 Feb 17;52(1):239. doi: 10.1007/s11033-025-10361-1. Mol Biol Rep. 2025. PMID: 39961936 Review.
-
Genomic and proteomic biomarkers for cancer: a multitude of opportunities.Biochim Biophys Acta. 2009 Dec;1796(2):176-93. doi: 10.1016/j.bbcan.2009.04.004. Epub 2009 May 4. Biochim Biophys Acta. 2009. PMID: 19406210 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0140-6736(05)66544-0', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0140-6736(05)66544-0'}, {'type': 'PubMed', 'value': '15894097', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15894097/'}]}
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.ejca.2006.10.020', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.ejca.2006.10.020'}, {'type': 'PubMed', 'value': '17223541', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17223541/'}]}
- Demonty G, Bernard-Marty C, Puglisi F, Mancini I, Piccart M (2007) Progress and new standards of care in the management of HER-2 positive breast cancer. Eur J Cancer 43:497–509 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '7266969', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7266969/'}]}
- Boccardo F, Bruzzi P, Rubagotti A, Nicolo GU, Rosso R (1981) Estrogen-like action of tamoxifen on vaginal epithelium in breast cancer patients. Oncology 38:281–285 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '6798066', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/6798066/'}]}
- Helgason S, Wilking N, Carlstrom K, Damber MG, von Schoultz B (1982) A comparative study of the estrogenic effects of tamoxifen and 17 beta-estradiol in postmenopausal women. J Clin Endocrinol Metab 54:404–408 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1023/B:BREA.0000003957.54851.11', 'is_inner': False, 'url': 'https://doi.org/10.1023/b:brea.0000003957.54851.11'}, {'type': 'PubMed', 'value': '14692654', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14692654/'}]}
- Ellmen J, Hakulinen P, Partanen A, Hayes DF (2003) Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res Treat 82:103–111 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

