Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects
- PMID: 17913310
- PMCID: PMC2243220
- DOI: 10.1016/j.vaccine.2007.08.042
Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects
Abstract
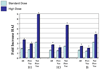
To improve immune responses to influenza vaccine, a trivalent inactivated vaccine containing 60 microg of the HA of each component (A/H3N2, A/H1N1, B) was compared to a licensed vaccine containing 15 microg of the HA of each. More local and systemic reactions were reported by subjects given the high dosage but only local pain and myalgias were significantly increased. The high dosage vaccine induced a higher frequency of serum antibody increases (> or =4-fold) in both hemagglutination-inhibiting (HAI) and neutralization tests for all three vaccine viruses in the total group as well as subjects vaccinated and those not vaccinated the previous year. Mean titers of antibody attained, the magnitude of antibody increases and the frequencies of persons with final HAI antibody titers > or =1:32, > or =1:64, and > or =1:128 were all greater for the high dosage group in both serologic tests, for all groups, and for all vaccine viruses. These increased immune responses should provide increased protection against influenza in the elderly.
Conflict of interest statement
Conflict of interest: Robert B. Couch has served as a consultant to GlaxoSmithKline, Vaxinnate and Dynavax.
Rebecca Brady receives funding to conduct clinical research studies from GlaxoSmithKline.
Robert Edelman is a consultant to Acambis, Inc.
Robert Belshe is a consultant to Merck and Medimmune, and is a speaker for Merck, Medimmune and sanofi pasteur.
Jose Capellan is an employee and investor of sanofi pasteur
Fred Ruben is an employee and investor of sanofi pasteur
Figures

Comment in
-
Influenza vaccine dosages.Vaccine. 2008 May 2;26(19):2305-6. doi: 10.1016/j.vaccine.2008.03.007. Epub 2008 Mar 27. Vaccine. 2008. PMID: 18407384 No abstract available.
Similar articles
-
Clinical trial to evaluate the safety and immunogenicity of a trivalent surface antigen seasonal influenza vaccine produced in mammalian cell culture and administered to young and elderly adults with and without A(H1N1) pre-vaccination.PLoS One. 2013 Aug 16;8(8):e70866. doi: 10.1371/journal.pone.0070866. eCollection 2013. PLoS One. 2013. PMID: 23976960 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial.JAMA. 2007 Apr 11;297(14):1577-82. doi: 10.1001/jama.297.14.1577. JAMA. 2007. PMID: 17426277 Clinical Trial.
-
Immunogenicity and safety of an inactivated 2012/2013 trivalent influenza vaccine produced in mammalian cell culture (Optaflu®): an open label, uncontrolled study.Hum Vaccin Immunother. 2014;10(2):441-8. doi: 10.4161/hv.27140. Epub 2013 Nov 15. Hum Vaccin Immunother. 2014. PMID: 24240428 Free PMC article. Clinical Trial.
-
Intradermally-administered influenza virus vaccine is safe and immunogenic in healthy adults 18-64 years of age.Vaccine. 2013 May 1;31(19):2358-65. doi: 10.1016/j.vaccine.2013.03.008. Epub 2013 Mar 13. Vaccine. 2013. PMID: 23499604 Clinical Trial.
-
Review of 10 years of clinical experience with Chinese domestic trivalent influenza vaccine Anflu®.Hum Vaccin Immunother. 2014;10(1):73-82. doi: 10.4161/hv.26715. Epub 2013 Oct 8. Hum Vaccin Immunother. 2014. PMID: 24104060 Free PMC article. Review.
Cited by
-
Immunogenicity and safety of high-dose trivalent inactivated influenza vaccine compared to standard-dose vaccine in children and young adults with cancer or HIV infection.Vaccine. 2016 Jun 8;34(27):3141-3148. doi: 10.1016/j.vaccine.2016.04.053. Epub 2016 Apr 26. Vaccine. 2016. PMID: 27129426 Free PMC article. Clinical Trial.
-
Roadmap for Sex-Responsive Influenza and COVID-19 Vaccine Research in Older Adults.Front Aging. 2022 Feb 11;3:836642. doi: 10.3389/fragi.2022.836642. eCollection 2022. Front Aging. 2022. PMID: 35821800 Free PMC article. Review.
-
Considerations for a Respiratory Syncytial Virus Vaccine Targeting an Elderly Population.Vaccines (Basel). 2021 Jun 9;9(6):624. doi: 10.3390/vaccines9060624. Vaccines (Basel). 2021. PMID: 34207770 Free PMC article. Review.
-
Double dose vs. standard dose influenza vaccination in patients with heart failure: a pilot study.Eur J Heart Fail. 2013 May;15(5):560-4. doi: 10.1093/eurjhf/hfs207. Epub 2013 Jan 4. Eur J Heart Fail. 2013. PMID: 23291729 Free PMC article. Clinical Trial.
-
Infection prevention and the medical director: uncharted territory.Clin J Am Soc Nephrol. 2015 May 7;10(5):863-74. doi: 10.2215/CJN.06050614. Epub 2015 Feb 20. Clin J Am Soc Nephrol. 2015. PMID: 25710803 Free PMC article. Review.
References
-
- Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–1836. - PubMed
-
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people; a systematic review. Lancet. 2005;366:1165–1174. - PubMed
-
- Farnik J, Bruj J. An outbreak of influenza A2 in a population with a known antibody profile. J Infect Dis. 1966;116:425–428. - PubMed
-
- Couch RB. An Overview of Serum Antibody Responses to Influenza Virus Antigens. In: Brown F, Haaheim LR, Wood JM, Schild GC, editors. Developments in Biologicals. Vol. 115. Karger: Laboratory Correlates of Immunity to Influenza; 2003. pp. 25–30. - PubMed
-
- Couch RB, Kasel JA. Immunity to influenza in man. Ann Rev Microbiol. 1983;37:529–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 RR023250/RR/NCRR NIH HHS/United States
- N01-AI-25461/AI/NIAID NIH HHS/United States
- N01 AI025461/AI/NIAID NIH HHS/United States
- M01 RR000059/RR/NCRR NIH HHS/United States
- N01-AI-25464/AI/NIAID NIH HHS/United States
- M01 RR016500/RR/NCRR NIH HHS/United States
- K12-RR-023250/RR/NCRR NIH HHS/United States
- N01 AI025464/AI/NIAID NIH HHS/United States
- M01-RR-16500/RR/NCRR NIH HHS/United States
- M01-RR-00059/RR/NCRR NIH HHS/United States
- N01 AI065298/AI/NIAID NIH HHS/United States
- N01 AI030039/AI/NIAID NIH HHS/United States
- N01 AI025459/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

